Today’s blog post is a Valentine’s Day message from our universe, sent 7500 years ago from an address 44,000,000,000,000,000,000 (that’s 44 quadrillion, or 4.4×1019) miles away.

As a scientist, I have always been intrigued the idea of spectroscopy—the idea that the different colors (wavelengths) of light reveal the nature of the universe around us. I even wrote another blog post about the chemistry of color after an inspiring trip to Alaska, “Bob’s Beautiful Blue Glaciers.”
Many scientists use spectroscopy in some form on a daily basis. In the CSN, we use spectroscopy to learn about the chemical makeup of nanoparticles, of different solutions, and pretty much everything. But spectroscopy isn’t just for studying chemicals in the lab. While I’m a chemistry professor by day, I’m an amateur astronomer by night, and one of my favorite activities is exploring how to use spectroscopy to image and understand the natural world that is visible above our heads. Our universe is filled with planets, comets, and nebulae — glowing remnants of long-ago exploded stars, and wispy trails of dust particles (including nanoparticles!) that eventually coagulate and give birth to new planets and new stars. All going on above our heads every day.

You might be wondering, how could we get chemical information from objects that are so far away? One of the unique features of spectroscopy is that different chemical elements absorb light and emit light at specific wavelengths, dictated by the laws of quantum mechanics. The wavelengths are unique for each element because their configuration of electrons is unique. For hydrogen atoms, it’s very simple since there is only one electron:

In a nebula, the gases are able to emit light because they are first excited by energy from a nearby star. Stars emit light at all wavelengths, including ultraviolet light and x-rays that are energetic enough that that they can excite electrons in many different elements. With hydrogen, starlight can excite the electrons from their natural resting state (scientists call that the 1s, or “ground state”) to higher levels, like the 2p level. When electrons drop back down from the 2p level to the 1s level, they emit light corresponding to the energy difference. Atoms made from different elements emit light at different wavelengths.
My colleague Cathy Murphy made an example diagram of this for her blog post on “Electrocuting a Pickle”:
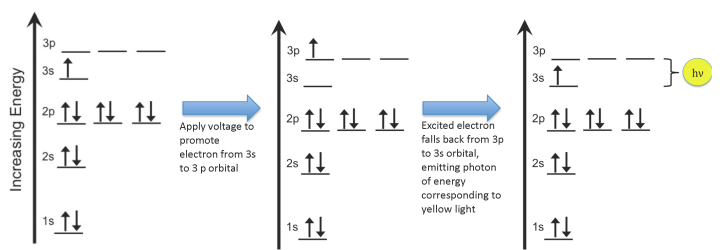
Put table salt (sodium chloride) in a flame and the heat of the flame excites the electrons in the sodium atoms, but when they drop back down they give off yellow light, at a wavelength of 589 nm. If you supply energy to hydrogen atoms, the electrons push up in energy, and when they fall back down they emit light with a wavelength of 656.3 nm: a nice deep red color. Oxygen atoms can get so excited that they lose two electrons (the atoms become ionized), but the remaining electrons give off light that is a beautiful bluish-green with a wavelength of 507 nm. On earth, these oxygen ions also create the greenish color of the glowing Aurora Borealis, or “Northern Lights”.

Chemical Images
When I want to get an image of a nebula (a region of glowing gas and dust), I can use special camera filters to focus on the wavelengths of light that I know are emitted by certain chemical elements – in this case hydrogen and oxygen. To make the images, I set up my telescope with a camera and a red filter (an “H-alpha” filter) that passes light only at the wavelength near 656.3 nm that hydrogen emits at. I then record and accumulate images over several hours and combine these into a single chemical image showing only the hydrogen. I add up many images (each a few minutes long) because a single snapshot can’t capture enough light to make the image, but that means I also have to use a special “equatorial” mount that keeps the telescope centered on the nebula as the earth rotates over time. I then do the same thing using an oxygen filter, creating a chemical image showing only oxygen. Finally, by combining the hydrogen and oxygen images, one can make a color image that shows the location of the different elements relative to one another. The relative intensities of red, green, and blue (3 different elements) are completely arbitrary in these kinds of images, so the overall color balance just depends on what kind of representation one wants to have.
Below are a few images I took showing this process with an object called the “Heart Nebula” (more officially, IC1805). The first looks only at hydrogen, the second only at oxygen, and the third shows a combination image with hydrogen presented as red, and oxygen presented as blue and green; these choices are close to the actual colors your eye would see directly. Regions that are red are mostly hydrogen, while regions that are blue-green are mostly oxygen, and regions that are white contain both. The very small white specks throughout the images are stars that lie between us and the nebula—not part of the nebula itself.

The nebula looks very different if you filter for wavelengths of light that are emitted by more elements, or if the different elements are presented at different levels of brightness.

While the Heart Nebula is huge (it is about 165 light-years wide – that’s 70,000,000,000,000 miles, or 50 times wider than our solar system), it’s still mostly empty space, with some individual atoms like hydrogen and oxygen. The energy to make these atoms glow comes from the very bright stars near the very center of the heart.
But look more closely, especially near the blue-white region near the center of the heart, and you’ll see wispy “dust lanes” – darker regions that formed from particles of interstellar dust that block light from the glowing hydrogen and oxygen behind them. What’s in the dust ?? Why, nanoparticles! And bigger particles too, of course. Nebulae like the Heart Nebula are often remnants of stars that have exploded… but they are also a place where gravity tugs on atoms of hydrogen, oxygen, carbon, silicon, and other elements, making them bump into onto another and form small molecules, which then coagulate together into nanoparticles on their way to forming larger agglomerates and eventually re-birthed stars and planets, in a continuing life cycle of birth and death in the universe around us.



