You could say that baking runs in my family: the inventor of angel food cake was a distant cousin, the big question at family gatherings when I was a kid was whether my grandfather made a cheesecake, and holidays are always full of homemade cookies, quick breads, and pies. One of my favorite food traditions is the cake baked in a lamb-shaped mold, an Easter tradition from the Polish side of my family.

For years after an incident in my childhood in which my oven mitt caught fire, I avoided doing anything requiring a stove or an oven. But I eventually overcame that and developed an interest in baking! I love baking cakes and experimenting with the flavor combinations. Why make the same old standards you can get at the grocery store? Half the fun is in trying different combinations, like chocolate and orange, almond and raspberry, or Earl Grey, orange, and white chocolate (the last one was an idea I borrowed—I sadly had not thought of it!). Once, a grocery order gave me six mangoes instead of one, so I used them in a sponge cake for a department picnic!
The toughest part of cakes is the frosting, which can be too melty, too sweet, too greasy, you name it! Get the taste and texture just right, and everyone will rave about it; get it wrong, and there will be a story to tell future generations. It helps to start with a good recipe. The weather is important, too—just about any frosting will hold up in a cool room, but if there’s any heat involved in the transport or the venue, some frostings will melt.

Here are a few types of frostings I have made with pictures of the final products and a table of their features:

Different types of frostings have qualities that make them suited for different uses. I definitely would not try to cover a whole cake with royal icing, nor would I use buttercream to try to glue gingerbread together. Beyond these functions, I never thought about the purpose of frosting until I studied cell membranes (specifically plasma membranes): Frosting protects the cake just like cell membranes protect cells. For example, have you ever left an unfrosted cake out on the counter for a few days? It dries out. One of the reasons we frost cakes is to keep the moisture in.
How does this work? Let’s look at the major components that make up both frosting and cell membranes:
Carbohydrates (sugar)
Sugar is one of the largest components of frosting. Without sugar, there would not be much to frosting, and it would have a terrible taste!† Sugar also provides some structure to frosting. In cell membranes, sugar chains called oligosaccharides are attached to the surface of lipids or proteins.1 In some types of cells, they wrap around the membrane to form a sort of protective coating. They also allow cells to detect each other. Here are the structures for sucrose (the table sugar often used in frosting) and an oligosaccharide (a sugar molecule you might see on a cell membrane):
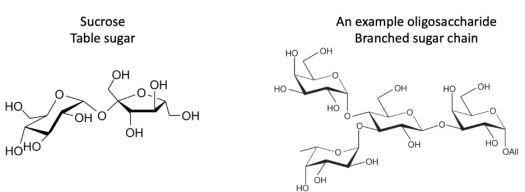
A quick primer on organic molecules like these sugars: each bend or unlabeled end of a line is a carbon atom with enough hydrogens to make four bonds. The “O”s are oxygen atoms, and the “H”s are hydrogen atoms. This style of diagram is meant to represent how the molecules look in 3D: imagine the thicker lines of the skewed hourglass shapes coming closer to you, and the thinner lines of the same shapes toward the back.
Lipids (fat)
Did you know there is a difference between frosting and icing?2 Frosting (including buttercream) contains fat, which makes it thick and gives it a rich flavor. Icing has no fat and dries into a shell-like coating. A glaze is a thin icing that is poured over the cake. Sugar is hygroscopic, which means it absorbs water from the air, so icings will soften over time in a humid environment. Frostings withstand humidity better because lipids like oil and butter act as a barrier against water. About half of a cell membrane is made of phospholipids (two fatty acids and a phosphate), which form two layers with the molecules’ water-repellant heads facing the water inside and outside of the cell.2 Small molecules like oxygen can pass through the lipid layers into and out of the cell, but ions and larger molecules need a different way of entering the cell, which I’ll talk more about soon.
Here are the structures for palmitic acid (one of the most abundant fats in butter)3 and a phospholipid you might see in a membrane, and an illustration of a membrane made of phospholipids:

Many frostings contain butter, but anyone who has had cake in a warm room or the summer heat knows how quickly a cake can turn into a melty disaster! Swapping out the butter (even partly) with shortening, which melts at a higher temperature, can prevent melting. The other ingredients make a difference, too! For a fun read about which frostings can withstand the heat, check out this article. How does this relate to cell membranes? They depend on how solid or liquid their lipids are, too! Turns out they must be slightly melted to perform their many functions, like moving molecules in and out of the cell and sending signals. My doctoral research was all about how nanoparticles affect the arrangement of lipids in membranes, but that’s a topic for another post.
Proteins (egg and dairy)
Frostings with dairy have a tiny amount of protein, and those with egg whites (meringue buttercream, “cooked” frosting, and royal icing) have a little more protein. Protein provides structure: if you whip egg whites for long enough, a stiff meringue develops. Protein increases the stability of frosting against heat if it’s cooked to a sufficiently high temperature (like in Italian meringue buttercream), because heating protein beyond a certain temperature changes its structure (like cooking an egg). Heated to a lower temperature (like in Swiss meringue buttercream), it melts just as easily as frostings without protein because the protein structure hasn’t changed. It solidifies icings for structural decorations. It doesn’t require much, though; too much protein, and you end up with scrambled eggs!

In cell membranes, proteins have all sorts of roles. Some add structural support like in frosting. Others form channels through which only certain ions or molecules can enter or exit the cell.1 Here are the structures of albumin (a common protein in egg white) and a transmembrane protein you might see in a cell membrane:

Other components
It’s difficult to quantify the amount of air in frosting, but stirring and whipping the mixture incorporates air in the form of bubbles that leads to that delightfully fluffy texture. And, of course, there is some water as well, either added or contained in the other ingredients (butter, milk, and egg whites all have a large amount of water), but it’s difficult to measure just how much is in frosting. Anyone up for an experiment?
Frosting has a small amount of other ingredients like salt and extracts for flavor, but these don’t contribute significantly to the structure of the frosting. One other ingredient we haven’t talked about is cholesterol. It does not appear to have a strong impact on frosting, but it plays a very important role in cell membranes. Different kinds of cells will have specific amounts of cholesterol, which controls how solid or liquid the membrane is.
Putting it all together

Here is a diagram of a cell membrane. Notice the similarity to the above ingredients: It is about 50/50 (by mass) lipids and proteins.1 The proteins are interspersed among the lipids to provide structure and specific functions. Carbohydrates are also attached to the outside of some of the proteins.
Let’s see examples how the ratio of ingredients affected the frostings from the first set of images above. I approximated the ratio of fats, proteins, carbohydrate, and the remaining components (by mass) using the nutritional information of the major ingredients.4 I ignored minor ingredients such as salt or flavorings. I also included the average ratio of fats, proteins, and carbohydrates in cell membranes for comparison, though they vary significantly.1

The fats in the two buttercreams make them soft and rich, like in the cubic cake and the lamb cake above. Royal icing and glaze have no fats, and as a result, they dry into a stiff solid and have a simple “sweet” flavor. These icings are great for gluing and for piping intricate decorations because they hold their shape very well. Cell membranes, by comparison, have a little more lipid (fat) compared to buttercreams. Without lipids in cell membranes, the membranes would be crunchy clusters of proteins and carbohydrates that don’t bend or flex. Imagine how fragile our cells would be if the membranes could not bend!
Proteins have a much greater role (and quantity) in cell membranes than in frosting because they control which molecules enter, exit, and interact with the cell. If present at all in frosting, all they do is provide a little structure. After all, cakes have one job (to be delicious… and hopefully look good, too), while cells are little chemical factories that require many working parts (proteins).
Frostings and icings have much more carbohydrates than cell membranes do, because we want them to taste sweet. The sugar also forms a nice crunch on the outside of icings. Buttercreams may have just a slight crust on the outside and are otherwise soft under that crust. Notice how little carbohydrate there is in cell membranes. Carbohydrates form a sort of barrier in cell membranes, though not as much as the lipids. Since taste is irrelevant to cell membranes, there’s no need to use all that sugar we put into frosting.
At first glance, a cell membrane probably does not make you think of cake frosting. However, they actually have quite a lot in common! It might help to think of membranes as a sort of complex frosting with protein gates that let molecules in and out, plus some carbohydrates that tell other cells and molecules what kind of cell it is.
And now I want to go bake and frost a cake. Bon appétit!
References
- Cooper, G. The Cell: A Molecular Approach (2nd ed). Sunderland (MA): Sinauer Associates; 2000. ISBN-10: 0-87893-106-6
- Gutoskey, E. What’s the Difference Between Frosting and Icing? Mental Floss, Jan 18, 2022. Retrieved from https://www.mentalfloss.com/article/653343/frosting-vs-icing-what-is-difference
- Lindmark Månsson, H. Fatty acids in bovine milk fat. Food & Nutrition Research, 2008 (52). DOI: 10.3402/fnr.v52i0.1821
- Fatsecret.com – frosting nutritional info retrieved from https://www.fatsecret.com/calories-nutrition/search?q=frosting
Footnotes
* By the way, if you guessed the cubic cake above is made to look like a Borg cube from Star Trek, you are correct! I didn’t color it like a Borg cube because gray dyes look good in photos but not so good on a plate!
† For recipes that call for powdered sugar or confectioner’s sugar, carefully check the sugar package that there is no starch in it. Sometimes cornstarch is added to prevent sticking, but it adds an unpleasant flavor
