The size of the materials in nanotechnology is one of the most important factors that power and define the properties of the nanomaterials. But there are many different techniques for measuring that size, and depending on the measurement methods, the results may vary! It doesn’t mean the actual lengths or diameters of the materials change: usually it means that in a certain method, we need to consider other factors that can affect the size measurement. Sometimes those factors cannot be ignored, especially when they can make a difference for the environmental impacts of the material. In the Center for Sustainable Nanotechnology, the dynamic light scattering (DLS) spectrophotometer is the instrument we use often because it’s relatively easy to use and gives us results fast. One distinct characteristic of DLS is that it can measure the size of particles that are suspended in solvent, like the tiny particles of mud you might see clouding a glass of dirty water. But one thing we need to remember is that it can sometimes tell us that the particles are bigger or different than they actually are.
Why is it so important to measure particles in suspension? A lot of ecosystems involve particles suspended in water, like lakes, rivers, and streams. Understanding the chemistry that happens when nanomaterials are exposed to an ecosystem is a major research goal for the Center for Sustainable Nanotechnology. To investigate this, researchers often study nanoparticles that are suspended in “biologically relevant aqueous media.” (That just means a liquid with some characteristics of real-world biology.) In this suspension, we can use the DLS spectrophotometer to measure the size of the nanoparticles, which helps us predict their interactions with biological organisms. However, the size obtained from DLS is usually different from the actual physical size of the particles that we know from measuring them before. Why is this happening? It is definitely not that the particles inflate in liquid! (Sometimes they do, but that is another story…)

The size discrepancy is deeply related to the way the DLS instrument calculates the size of the particles. It starts from understanding Brownian motion: the random movement of the suspended particles in a fluid. (Knowing how Brownian motion works, we can assume that the suspended particles move around in liquid at a certain rate freely and independently. Smaller particles move faster, and larger particles move more slowly.) But how can the DLS instrument measure the size of the materials when they are constantly moving?
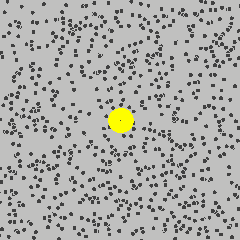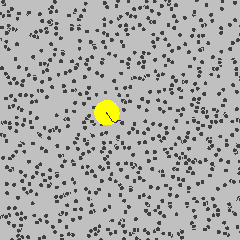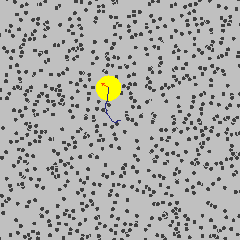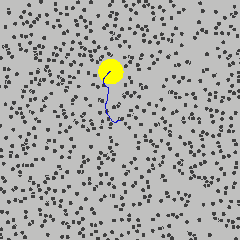
Actually, the instrument is not capturing or recording the physical properties of the suspended nanomaterials. Instead, it uses a laser to illuminate the particles, and then collects information about the intensity fluctuation of the light that scatters when the laser hits the particles. This scattering occurs in all directions, and its intensity fluctuation depends on the size and speed of the suspended nanoparticles. (To learn more about this type of light scattering, check out this 90 second video from LibLab)

So if light scattering is so useful for calculating particle size, why isn’t the DLS always completely accurate? Well, there are many factors which can affect the size results from DLS. First, the solvent where the particles are suspended is made of its own molecule: H2O for water and C6H12 for cyclohexane. The solvent molecules near the surface of the nanoparticles interact with the nanoparticles, sometimes tightly binding to the surface and moving along with the particles. (This is kind of like when Styrofoam peanuts stick to a cat). During the measurement, the instrument assumes that this layer of solvent stuck to the nanoparticle surface is also a part of the particle, so it measures a larger size than the actual physical size of the particles.

In some cases, we can unstick some of this solvent layer from the nanoparticle surface. For example, if we add salt to the solution (like sodium chloride, which is table salt), it can thin down the solvent layer so the DLS measures a smaller size. This is why when we measure the particle size via DLS, we seriously and carefully consider the solvent composition and other parameters, such as temperature, that can affect the size that is measured.
Some nanoparticles have extra molecules called ligands stuck to their surface to give them certain chemical or physical properties, such as making them stably suspended (not clumped together) in a certain liquid. These ligands are really hard to see in a microscope and sometimes researchers forget about them when we are making size measurements. Some ligand molecules can be long, and depending on the chemical interaction between the ligand and the solvent molecules, the ligand molecules stretch themselves to the solvent media or shrink themselves, like a coil, and form a mushroom-like structure.

Of course, these ligands will make a difference in DLS measurements. When the particles are dispersed in a certain medium whether the ligands will stretch out or shrink depends on the chemical properties of the solvents and the ligand molecules. So, we need to take these into consideration when we use DLS to measure the size of particles with ligands.
Another challenge is that in DLS measurements, one big assumption is that the nanoparticles are spherical, which is not true in many cases. Imagine a batch of gold nanorods which are 50 nm long and 10 nm wide. This shape can make the DLS instrument interpret that the particles are spheres 50 nm across!

As you can imagine, if the rods get longer, it will hugely affect the diffusion rate measurement from the instrument. However, if the rods get wider in diameter, it would not make as big a difference. This means that, unlike other types of measurement, the DLS instrument is great for measuring spherical particles, but it may not be appropriate for detecting small changes in shape for non-spherical particles.
After talking about all of these limitations of DLS, you might wonder why we use it! But it is still quite useful when we want to quickly study colloidal stability (whether particles get aggregated or even make sediment), and diameter sizes of the suspended particles. This is all important for understanding possible environmental impacts of the nanoparticles because, depending on their sizes and chemical states, the nanoparticles in suspension interact with various organisms very differently. With proper understanding of the principles behind the technique, DLS can provide necessary information for scientists like me who do research about nanoparticles in liquid.
EDUCATIONAL RESOURCES
- Khan Academy: Scattering of light & Tyndall effect (video by Mahesh Shenoy)
- TryNano.org: What is a nanometer? (lesson plan ages 8-11)
REFERENCES
- Lin, Y.-S.; Haynes, C. L. Impacts of Mesoporous Silica Nanoparticle Size, Pore Ordering, and Pore Integrity on Hemolytic Activity. Journal of the American Chemical Society. 2010, 132 (13), 4834–4842. Doi: 10.1021/ja910846q
- Abstiens, K.; Gregoritza, M.; Goepferich, A. M. Ligand Density and Linker Length Are Critical Factors for Multivalent Nanoparticle–Receptor Interactions. ACS Applied Materials Interfaces 2019, 11 (1), 1311–1320. Doi: 10.1021/acsami.8b18843
- Schulz, F.; Dahl, G. T.; Besztejan, S.; Schroer, M. A.; Lehmkühler, F.; Grübel, G.; Vossmeyer, T.; Lange, H. Ligand Layer Engineering To Control Stability and Interfacial Properties of Nanoparticles. Langmuir 2016, 32 (31), 7897–7907. Doi: 10.1021/acs.langmuir.6b01704
- Carnovale, C.; Bryant, G.; Shukla, R.; Bansal, V. Identifying Trends in Gold Nanoparticle Toxicity and Uptake: Size, Shape, Capping Ligand, and Biological Corona. ACS Omega 2019, 4 (1), 242–256. Doi: 10.1021/acsomega.8b03227
