co-written by Claire Alford
People love to categorize themselves by personality type, whether it’s describing what kind of baked potato we are, or which character we have most in common with from Stranger Things. But have you ever wondered what kind of nanoparticle you would be? We thought it would be fun to create a Buzzfeed quiz to relate science-based nanoparticle properties to non-scientific personality traits. Given that there is so much variation across types of nanoparticles, we created a type of nanoparticle zodiac. After you’ve answered the questions, the quiz will tell you the nanoparticle that you are most similar to. We only picked ten nanoparticles for this quiz, and hopefully it goes without saying that although the nanoparticle descriptions are based on science, the personality part of this is purely for entertainment. Go take the quiz (it is short and fun!), then come back to learn more about your nanoparticle (and the others too)!
Disclaimer: In a scenario where you are equally two or more nanoparticles, Buzzfeed automatically assigns you as the first nanoparticle from the list.

The Nanoparticles
Silver Nanoparticles (Ag)1,2,3
When it comes to silver nanoparticles (Ag NPs), you may have heard that they are found in laundry detergent, silverware, and clothing. Ag NPs are used in these applications because they are known to exhibit antibacterial, antiviral, and antifungal properties. Ag NPs can be considered a “jack-of-all-trades” in that they have the abovementioned properties as well as anti-inflammatory and anticancer uses. Because of Ag NPs’ unique properties (i.e., optical, electrical, thermal, and biological), they also have potential use in the biomedical, food, and industrial sectors. Interestingly, researchers have found that Ag NPs can be synthesized by ordinary life forms like bacteria. This is one of the ways that researchers can get Ag NPs more efficiently via green synthesis methods as opposed to conventional chemical routes that are more costly and produce hazardous byproducts. These nanoparticles exhibit localized surface plasmon resonance (LSPR), which is a small-scale version of surface plasmon resonance (SPR; discussed below). Scientists can use LSPR to learn about the shape of Ag NPs and their surrounding environments.
If you are a silver NP:
- You are a protector: these nanoparticles are used in detergents, silverware, and clothing for protection against pathogens
- You are a jack-of-all trades: these particles have lots of different properties
- You are environmentally friendly: scientists use “green chemistry” to synthesize silver NPs
- You’re great at leading small groups, and may have short-lived energy: LSPR suggests that you are better at leading small groups of people, not larger groups like SPR. You may also have short-lived bursts of energy during the day
- You are a reflective person: Ag NPs can be used to coat glass for making mirrors and, given our Moon’s associated esoteric beliefs to be a symbol of inner reflection (i.e., a mirror), Ag NPs are associated with the Moon
Aluminum Oxide Nanoparticles (Al2O3)4,5
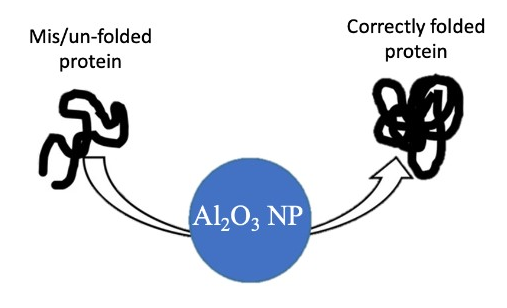
Aluminum oxide nanoparticles (Al2O3 NPs), also known as alumina, are very hardy due to their ability to withstand high temperatures and physically wearing environments. This makes sense given that they are the starting material for making rubies and sapphires. In addition, they are poor electrical conductors, which makes them different from other metal oxide nanoparticles. Some researchers have investigated the use of Al2O3 NPs for treating asthma, while others use them to prevent proteins from folding incorrectly (folding errors can cause problems with protein function). One variety of Al2O3 NPs was shown to reduce the concentration of antibiotics in hospital wastewater. Such promising results indicate that Al2O3 NPs might be useful for preventing antibiotic resistance in pathogens.
If you are an Al2O3 NP:
- You are a lifesaver: these NPs are used to treat asthma and provide soothing energy
- You are resilient: these NPs are hardy and have learned important life lessons from hardship
- You guide others toward solutions during times of conflict: Al2O3 NPs prevent protein misfolding/aggregation which helps the protein take on its correct form
- Your environment does not bring you down: Al2O3 NPs can push through difficult conditions
- You work best under pressure: Al2O3 NPs can withstand high temperatures and wearing environments, they are akin to people that thrive in high-pressure environments
Gold Nanoparticles (Au)6,7,8
Despite gold having been one of the first metals ever discovered, gold nanoparticles (Au NPs) were not studied by modern scientists until 1971. Au NPs have a very wide range of biomedical applications ranging from biosensors and bioimaging to targeted delivery of drugs to cancer cells. Given gold’s inert nature, it is helpful for delivering payloads of medicine in the body because the gold itself won’t harm living tissues (it is biocompatible). Another property of Au NPs is surface plasmon resonance (SPR), which is discussed in detail in this blog post; basically it describes that the electrons of Au NPs can be vibrated using specific wavelengths of light. This SPR property of Au NPs allows them to be used for disrupting tumor cells and essentially killing them in a process known as photothermal therapy. Because of all of this, Au NPs have already had a major impact on medicine.
Au NPs have also demonstrated promise for applications in other fields, such as in energy harvesting and water purification. As Au NPs get smaller and smaller, their properties change, which opens up opportunities for even more technological uses. For example, if a Au NP’s diameter is within the range of 1-2 nm, particles that were generally inert now become a great catalyst for converting carbon monoxide into carbon dioxide, much like the catalytic converter of a car. Also, as the diameter of Au NPs change, they absorb slightly different wavelengths of light. Due to this property, Au NPs hold potential in improving current solar cell technology by absorbing a greater array of light wavelengths. These slight changes in diameter also contribute to the color that is observed, from red to magenta, in colloidally stable solutions of Au NPs.
If you are a gold nanoparticle:
- You are great at leading large groups: the SPR quality of Au NPs correlates to a person who can lead a large group in concerted action
- You de-escalate conflicts to decrease tension in difficult situations: gold NPs can work as catalysts that convert CO to CO2
- You are a confidant: gold NPs quench fluorescence, which is similar to a friend who can listen to your emotions and act as a shoulder to cry on
- You enjoy photography: Au NPs are known to be used in bioimaging

Carbon Dots9,10
Carbon dots (CDs) can be prepared from a plethora of organic (carbon-based) materials such as honey, coffee, orange peel, and tree bark – the list goes on and on. Given CDs’ “green” starting reagents, it is no surprise that CDs have great biocompatibility and are generally considered eco-friendly. Another trait of CDs is their unique optical properties. They exhibit photoluminescence, which means these nanoparticles absorb a specific wavelength in the visible region and emit light at a different wavelength. This property of CDs, along with their biocompatibility, has made them a very important NP for bioimaging purposes. When comparing synthesis to other nanoparticles, CDs take the cake when it comes to affordability. Also, synthesis processes using different types of material or reaction conditions yield CDs with unique behaviors for absorbing and emitting light, so there is lots of variability in what they can do! (Researchers in the CSN even published a guide for how high school and college teachers can make carbon dots in a regular microwave!11
If you are a carbon dot:
- You enjoy painting as a hobby: CDs come in an array of colors and can be compared to a palette with multiple colors
- You are a minimalist: CDs can be made from simple and affordable starting materials
- You are a resourceful person: CDs are made from a range of super simple materials and can be used for lots of different things
- You value the time spent on meal prepping: Starting reagents of CDs are organic materials such as orange peels and coffee grounds

Carbon Nanotubes12
Carbon nanotubes (CNTs) are generally made of a single sheet of graphene, a famous nanomaterial comprising a 2-dimensional array of carbon atoms. The graphene sheet is exposed to a chemical process that closes it into a CNT. Among all the NPs on this list, the cylindrical structure of CNTs is unique. The diameter of the cylinders are what make CNTs “nano”: they range between 1-10 nm wide, while their lengths are in the micrometer range. Characteristics of CNTs include high thermal and electrical conductivity, along with high tensile strength and elasticity. Because of these properties, CNTs have been used to make bullet proof jackets and durable sporting goods. Bare CNTs are chemically neutral. However, when adorned with functional groups of molecules, they can work as catalysts. These surface modifications can also optimize their solubility and dispersion in liquids.
If you are a CNT:
- You are level headed: CNTs are generally known to be inert
- You spend most of your free time exercising: CNTs have been used to make sports equipment
- You stay more focused on your long term goals than short term: CNTs’ cylindrical structure is like a long tunnel that blocks out distractions as you work toward your long term goals
- You like the excitement of living life on the edge: CNTs’ long, thin structure is like a tightrope

Cerium Oxide Nanoparticles (CeO2/Ce2O3)13, 14, 15, 16
Cerium Oxide NPs (CeO2 NPs), also known as nanoceria, are composed of the most common rare earth element, cerium. These rare earth elements are essential for the functionality of electronic devices such as laptops and cellphones. CeO2 NPs have the unique ability to gain or lose electrons to switch Ce oxidation states from +3 to +4 charge, which gives these NPs catalytic activity. An example of nanoceria’s catalytic activity is in its antioxidant properties; they break down harmful reactive oxygen species such as hydrogen peroxide. On the other hand, nanoceria has also been shown to produce reactive oxygen through its oxidase activity (this essentially adds an oxygen to biological molecules, which can ultimately become reactive oxygen species). Researchers have also observed that CeO2 NPs possess anticancer activity and are effective antibacterial agents against bacteria. Depending on how they are made, CeO2 NPs have different effects on cells. Cells exposed to CeO2 NPs that were synthesized in pure water were less able to survive compared to cells exposed to CeO2 NPs that were made in polyethylene glycol (similar to the liquid used for making carbon dots, see image above). So controlling the way we make nanoceria can help us control its biocompatibility.
If you are a CeO2 NP:
- You are protective of your friends: CeO2 NPs have antioxidant activity that can protect organisms from the effects of harmful reactive oxygen species
- You prioritize self-improvement and personal growth from within: CeO2 NPs can change oxidation states
- You are a self-starter: CeO2 NPs are great catalysts that can initiate reactions between other materials
- You spend your free time working on your car: CeO2 is the most commonly used material for making catalytic converters

Copper Oxide Nanoparticles (CuO)17, 18, 19
Scientists have found that copper oxide nanoparticles (CuO NPs) can help deliver copper to plants as a micronutrient, and can also protect plants against fungus. One theory is that CuO NPs elicit this antifungal property in plants by boosting the plant’s natural defenses. CuO NPs have major potential for improving current agricultural practices as well as alleviating crop loss due to harmful fungus. Another area where CuO NPs are showing potential is in making antimicrobial fabrics in the biomedical field. In addition, researchers have been able to use different levels of acidity in synthesis to make different shapes of CuO NPs, including flat and/or spike-like. Unlike some other nanoparticles like gold or carbon dots, CuO NPs can be hazardous to human white blood cells at high concentrations, so they are not used in medical applications.
If you are a CuO NP:
- You enjoy gardening: CuO NPs can be used to deliver nutrients to plants
- You enjoy spending time with friends, but you value your alone time: CuO NPs are generally safe at low concentrations but can be toxic at high concentrations
- You want to leave a big impact on the world: CuO NPs have potential to alleviate food scarcity by increasing crop production
- Your environment sets your mood for the day: depending on the synthesis conditions, CuO NPs can have different morphologies (shapes)

Iron Oxide Nanoparticles (Fe3O4)20-24
How cool would it be to see nanoparticle properties with your own eyes? With iron oxide nanoparticles (Fe3O4 NPs), you can! Fe3O4 NPs actually have a property that is visibly discernible to the human eye: superparamagnetism. Superparamagnetism means the Fe3O4 NPs move toward magnets but aren’t magnetic if there’s no other magnet around. This property is significant to researchers because it makes these NPs easy to retrieve with a magnetic field, which is not possible with some other nanoparticles.

In the medical field, scientists are making strides in the “theranostic” use of Fe3O4 NPs. Theranostics is a field that combines therapy and diagnostics to achieve diagnosis and therapeutic aid at the same time. Fe3O4 NPs can be used to guide therapeutic payloads to specific tissues or cells while also highlighting the targeted cells in MRI imaging. Hyperthermia is another therapeutic technique involving the use of Fe3O4 NPs, and it is used to treat cancer. This process involves targeting Fe3O4 NPs toward the cancerous tissues, and then exposing them to an alternating magnetic field. This magnetic activity causes the NPs to heat up and kill the cancer cells while leaving normal cells intact. You can also find these nanoparticles rocking out to Mick Jagger on the weekends (just kidding).
If you are an Fe3O4 NP:
- You are a rolling stone: Fe3O4 NPs are superparamagnetic, so they are moved by outside magnetic fields
- You side with your closest friend amidst conflict in your friend group: Fe3O4 NPs stick with magnetic materials that attract them, like someone might stick with their best friend through thick and thin
- You naturally gravitate towards the right people for you when making new friends, like Fe3O4 NPs are drawn to magnets
- You are easily affected by your environment- Fe3O4 (magnetite) is easily oxidized to Fe2O3 (maghemite)

Silica Nanoparticles (SiO2)25,26
Sílice, or silicon dioxide, nanoparticles (SiO2 NPs) are the most widely produced nanomaterial, with a yearly global production of approximately 185-1,400 kilotons. To give some perspective, the annual global production of aluminum is about 6,230 kilotons. Certain forms of SiO2 NPs are used throughout society to improve health or make life more convenient. For example, in medicine they can be used to help carry drugs, and can even deliver their payloads specifically to cancer cells. Many of these medical applications utilize mesoporous SiO2 NPs, which are spheres with pores distributed across the nanoparticle surface – to visualize this shape, think of a golf ball or a whiffle ball. Powdered foods like pancake mix or powdered milk and soups may use SiO2 NP complexes to prevent the product from forming clumps. Who knew you could find nanoparticles in your favorite Sunday morning breakfast?

If you are a SiO2 NP:
- You absorb outside energies easily and bottle up your emotions: SiO2 NP have a mesoporous form that can absorb things easily and/or carry other substances inside
- You enjoy watching or playing golf: mesoporous SiO2 NPs resemble the shape of a golf ball
- You are most productive when things are orderly: SiO2 NPs can be used for separating mixtures into pure forms
- You are a very important person: SiO2 NPs are the most produced NP in society

Titanium Dioxide Nanoparticles (TiO2)27
Both everyday and futuristic applications are possible for titanium dioxide nanoparticles (TiO2 NPs). These biocompatible NPs are used as a pigment in paper because they reflect light so well they actually increase how bright the paper looks. TiO2 NPs are also reaction catalysts that boost chemical reactions in many technological and energy applications. For example, TiO2 NPs can catalyze the creation of hydrogen gas by splitting water molecules. As a result, these NPs hold much promise for creating hydrogen fuel for sustainable technologies. Also, TiO2 NPs can be used in solar panels, another form of sustainable energy. Scientists are also working on using them in lithium ion batteries. The applications of TiO2 NPs are centered on technologies and energy forms that will make the world more sustainable.
If you are a TiO2 NP:
- You have a bright and cheery personality: TiO2 NPs are used as a white pigment in paper making
- You like to play video games in your free time and are tech savvy: TiO2 NPs are used for technological applications
- You have a sweet tooth for candy and sweets: TiO2 NPs are used to enhance the bright white color of some candies and sweets*You get organized using electronic methods, such as digital calendars and reminders: TiO2 are being investigated for possible use in lithium ion batteries, which are currently used to power laptops and phones

Now that you’ve learned about all ten different kinds of nanoparticles, why not use your new insights to go back and take our quiz again?
REFERENCES
- alicehuang2000. “Comparing LSPR and SPR for Diagnostics – LamdaGen.” YouTube, YouTube, 13 Nov. 2015, https://www.youtube.com/watch?v=QLT1vrnJXWI
- Klaus T, et al. Silver-based crystalline nanoparticles, microbially fabricated. Proceedings of the National Academy of Sciences of the USA. 1999, 96(24):13611-4. doi: 10.1073/pnas.96.24.13611.
- Zhang XF, et al. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. International Journal of Molecular Science. 2016, 17(9): 1534. doi: 10.3390/ijms17091534.
- Dao TH, et al. Adsorption Characteristics of Synthesized Polyelectrolytes onto Alumina Nanoparticles and their Application in Antibiotic Removal. Langmuir. 2020, 36: 13001-13011. doi: 10.1021/acs.langmuir.0c02352.
- Hassanpour, P. et al. Biomedical applications of aluminum oxide nanoparticles. Micro Nano Letters, 2018, 13: 1227-1231. https://doi.org/10.1049/mnl.2018.5070
- Dykman LA, Khlebtsov NG. Gold nanoparticles in biology and medicine: recent advances and prospects. Acta Naturae. 2011, 3:34-55. PMID: 22649683.
- NatureVideoChannel. “Tiny Treasure: The Future of Nano-Gold.” YouTube, 28 Jan. 2015, https://www.youtube.com/watch?v=QorK2X7GsVU
- Yeh YC, Creran B, Rotello VM. Gold nanoparticles: preparation, properties, and applications in bionanotechnology. Nanoscale. 2012 4:1871-80. doi: 10.1039/c1nr11188d.
- Liu J, Li R, Yang B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Central Science. 2020, 6:2179-2195. doi: 10.1021/acscentsci.0c01306
- Yao B, Huang H, Liu Y, Kang Z. Carbon Dots: A Small Conundrum. Trends in Chemistry. 2019, 1:235-246. doi: 10.1016/j.trechm.2019.02.003
- Pham S. et al. Carbon Dots: A Modular Activity To Teach Fluorescence and Nanotechnology at Multiple Levels. Journal of Chemical Education, 2017 94 (8), 1143-1149 DOI: 10.1021/acs.jchemed.6b00995
- Cheap Tubes Inc. “Applications of Carbon Nanotubes.” AZoNano.com, 9 Aug. 2019, https://www.azonano.com/article.aspx?ArticleID=4842
- Biomedical Applications of Nanoceria,” YouTube, 25 Jan. 2022, https://www.youtube.com/watch?v=7BFdTvF8qnE
- Dutta D, et al. Green synthesized cerium oxide nanoparticle: A prospective drug against oxidative harm. Colloids and Surfaces B: Biointerfaces. 2016, 147: 45-53. doi: 10.1016/j.colsurfb.2016.07.045
- Johnson I. “Cerium.” Royal Australian Chemical Institute, 2011. https://raci.imiscloud.com/common/Uploaded%20files/Periodic%20files/409.pdf
- Karakoti AS, et al. Nanoceria as Antioxidant: Synthesis and Biomedical Applications. The Journal of The Minerals, Metals & Materials Society, 2008, 60(3):33-37. doi: 10.1007/s11837-008-0029-8 .
- Assadian E, et al. Toxicity of Copper Oxide (CuO) Nanoparticles on Human Blood Lymphocytes. Biological Trace Element Research. 2018, 184(2): 350-357. doi: 10.1007/s12011-017-1170-4
- Borgatta J, et al. Biomolecular corona formation on CuO nanoparticles in plant xylem fluid. Environmental Science: Nano. 2021, 8: 1067-1080. doi: 10.1039/D1EN00140J
- Grigore ME, et al. Methods of Synthesis, Properties and Biomedical Applications of CuO Nanoparticles. Pharmaceuticals. 2016, 9(4): 75. doi: 10.3390/ph9040075
- Ali A, et al. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnology, Science, & Applications. 2016, 9: 49-67. doi: 10.2147/NSA.S99986
- Liu G, Gao J, Ai H, Chen X. Applications and potential toxicity of magnetic iron oxide nanoparticles. Small. 2013, 9: 1533-45. doi: 10.1002/smll.201201531
- American Chemical Society. “Sopping up Sunblock from Oceans to Save Coral Reefs (Video).” American Chemical Society, 21 Aug. 2017, http://bit.ly/acssunblock
- Wahajuddin, Arora S. Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. International Journal of Nanomedicine. 2012, 7: 3445-71. doi: 10.2147/IJN.S30320
- Dheyab M, et al. Simple rapid stabilization method through citric acid modification for magnetite nanoparticles. Scientific Reports, 2020, 10: 10793. doi: 10.1038/s41598-020-67869-8
- Mebert AM, et al. Nanoengineered silica: Properties, applications and toxicity. Food and Chemical Toxicology. 2017, 109(Pt 1) :753-770. doi: 10.1016/j.fct.2017.05.054
- Yang X, et al. Distinguishing the sources of silica nanoparticles by dual isotopic fingerprinting and machine learning. Nature Communications, 2019, 10, 1620: . doi: 10.1038/s41467-019-09629-5
- Ali I, Suhail M, Alothman ZA, Alwarthan A. Recent advances in syntheses, properties and applications of TiO2 nanostructures. RSC Advances. 2018, 8(53): 30125-30147. doi: 10.1039/c8ra06517a
