With winter and our first snowfall right around the corner where I live in Wisconsin, I am really looking forward to playing in the snow! One of my favorite activities is to throw snowballs up in the air while on a walk with my dog, Ginny. She jumps to catch the snowball on its way back down and shreds it up, often getting a face full of snow in the process. This game is fun all by itself, but one day while we were playing I also noticed a connection between the process of making a snowball and synthesizing nanoparticles.

Now for a quick snowball tutorial so we’re all on the same page. When I start to make a snowball for my dog, I start by scooping up snow with both hands and compressing it together. This part is usually where I spend most of my time: the small snowball can crumble in my hands if I’m not careful, and then I have to start over. The type of snow does make a difference (humidity, size of flakes, etc.) but size seems to play a big role in how well the ball holds together. While my dog will be happy with any snowball, she really loves trying to catch bigger snowballs.
So, to make my snowball bigger, I roll it along in the snow so that it picks up snow along the outside and gets bigger. While I’m rolling it, I have to push it with my hands, and that inevitably knocks some of the new snow off where I touch it. Generally, though, once I get the snowball big enough, it rolls right over the chunks of my previous snowball attempts and picks them right up. Once it gets past a certain size, I don’t have to worry about it crumbling to pieces anymore. And now that I’ve got a bigger snowball, my dog’s tail starts wagging even faster and her eyes get wider with excitement.

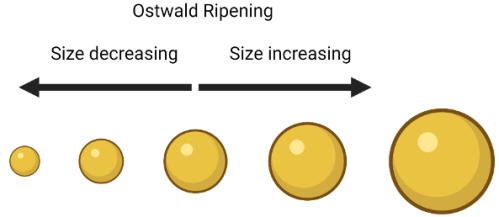
Setting my dog aside for now (sorry Ginny), let’s say we have a big snowball and a little snowball. The bigger snowball is more stable and less likely to fall apart when it is moving around. Why? Because there is more snow on the inside of the snowball holding it all together (the stuff on the inside can’t fall apart because the outside stuff is protecting it. Even if some snow comes off the surface of the big snowball, the snowball will still be pretty big. If that same amount of snow came off of the smaller snowball, it would get way smaller, and have even less snow holding it together. These two factors lead to the outcome that the larger snowball is more likely to keep getting bigger, while the smaller snowball is more likely to get smaller or fall apart altogether. I should note that this is just a relative comparison — of course a large snowball can fall apart and a small snowball can get bigger, otherwise we’d never be able to make good snowballs at all!

So what does this have to do with making nanoparticles? I’m happy to say it is through my favorite nanoparticle synthesis term: Ostwald Ripening. No, this isn’t ripening like a banana turning brown. At its most simple, Ostwald Ripening says that bigger nanoparticles tend to get bigger while smaller nanoparticles tend to get smaller, just like snowballs! Ostwald ripening is named after a Nobel Prize winning chemist, Wilhelm Ostwald, who first described small particles dissolving and then depositing on larger crystals in 1896.
To explain how this happens with a nanoparticle, let’s imagine shrinking down so that the nanoparticles look like snowballs to us. (To get an idea of what that would be like, read this blog post). Just like how snow can stick to a snowball or fall off of the snowball while we are rolling it, at this size we would see atoms sticking to the surface of the nanoparticle and atoms falling off the surface of the nanoparticle. Atoms can fall off the surface of the nanoparticle because they are not as “happy” as the atoms inside the nanoparticle. Atoms stick together tightly when they have lots of other atoms around them to connect to. On the surface of a particle, atoms can’t make as many connections because they only have other atoms on one side of them.
So atoms at the surface are sticking to and falling off of the particle, just like snow sticking to and falling off a snowball as we roll it. As the particles get bigger, the atoms falling off of the surface of the nanoparticle have less effect on the particle size, just like when snow comes off a larger snowball. That means that larger nanoparticles will tend to get bigger! Now the next time you roll a snowball, you’ll think of nanotechnology – you’re welcome!