I am a second-generation science fiction fan; my parents have been Star Trek fans since the ‘60s, and I grew up on PBS reruns of Doctor Who in the ‘80s. Like so many others, my appreciation for science fiction definitely played a role in developing my interests in real-world science. One of my favorite ways to celebrate this intersection is by attending CONvergence, an annual festival of all things geeky that takes place in the Twin Cities where I live.

To anyone who has attended a science fiction or other genre convention, it will probably come as no surprise that there is a strong overlap between people who love sci fi and fantasy and people who love science. One of the wonderful things about CONvergence is its deliberately broad and inclusive appeal: there is costuming, there is art, there is gaming; there are panels on comics, film, TV, novels, science, and history. Attendees can volunteer to help out with any facet of running the convention, as well as shop the dealer’s room and go to dozens of different themed parties. Throughout the weekend I saw plenty of babies, kids, teenagers, middle-aged people like me, and elderly folks too, with a refreshing cross section of gender and racial representation among attendees.

Since I have been attending CONvergence on and off for almost twenty years, I was especially pleased to be able to contribute this year in my professional capacity as the Director of Education and Outreach for the Center for Sustainable Nanotechnology. In addition to my usual fannish activities and volunteering, I got to participate in two panels and two demo activities directly related to science outreach.
The Panels
The first panel I participated in was “The Periodic Table at 150.” I gave the audience a quick rundown of the table’s organization (see this post for a review), and my co-panelist talked about some of the historical background for the table, including Mendeleev’s life story. I was able to borrow a couple of large periodic tables from our university Chemistry Department; we taped the largest one up on the wall behind us and referred to it frequently throughout the panel. The audience members had great questions and comments, which led us to chat about topics ranging from super-heavy elements to chemical poisons in fiction.

My second panel was called “Ask a Scientist,” and was exactly that: we had four scientists with experience in different fields (including physics, climate science, math, biology, pharmacy, cognitive science, and chemistry) and invited any and all questions from the audience. I had the chance to talk a little bit about some recent research from the CSN on sustainable battery materials,1 and got to learn from my co-panelists about everything from probiotics to proteins in human fossils to understanding calculus through population dynamics.
The Demonstrations
Our two science demonstrations were even more directly tied to the work of the Center for Sustainable Nanotechnology. The first one, “Nanoblocks,” involves demonstrating the importance of surface area-to-volume ratio in nanotechnology.2 Participants have a box full of fuzzy pompoms and two types of blocks: one large one about 3” on a side, and eight small ones about 1.5” on a side, all completely covered in Velcro. Both take up the same total amount of space (volume) but the group of smaller blocks has about twice as much Velcro as the big one (surface area).
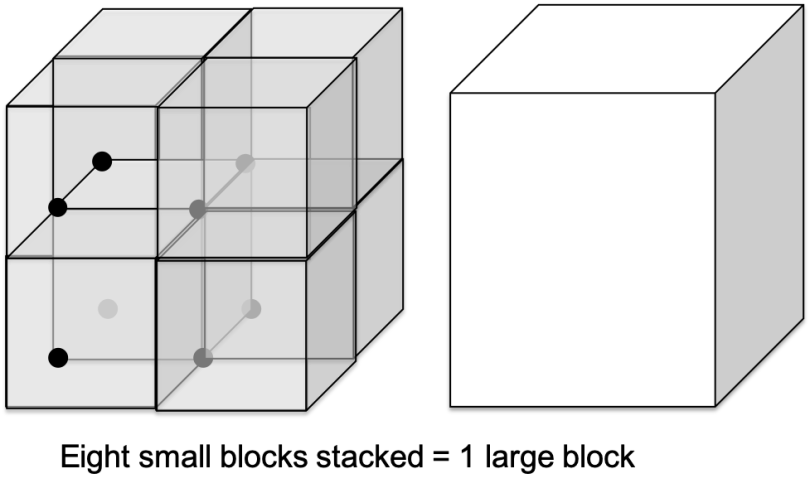
The idea is to imagine that the pompoms are pollutants in air or water, and the blocks are a material that can remove the pollution by sticking to it (this is called adsorption). Participants place either the one large block or the eight small blocks in a box with the pompoms, shake the box for a set amount of time, and then record how many pompoms have stuck to the Velcro. What do you think will happen?

If you’ve read a few of our blog posts, you could probably guess that the smaller blocks will do a better job of picking up the pompoms overall. At our demo last week we actually had one group who found the opposite result, so we spent a little time talking about whether variables other than surface area (such as differences in the types of Velcro on the blocks, the heaviness of the blocks, or the movements with which people were shaking their boxes) might have affected our results. Curiosity about unexpected findings is one of the joys of science!
Our second demonstration was called “Living Room Electron Microscopy.” It was developed by CSN graduate student Natalie Hudson-Smith and colleagues as a way to teach about Transmission Electron Microscopy (TEM) when you don’t have access to the real thing, and it was recently published in the Journal of Chemical Education.3 Natalie and her co-authors had piloted the activity last summer with a group of high school science campers, but this was the first time it had been presented since then. The setup involves model “microscopes” made of PVC pipes that have UV lights mounted at one end and a “sample” at the other end, with a sheet of UV-sensitive cyanotype paper at the bottom.

The UV light shines over the sample (in this case beads, buttons, and even a few pompoms from the nanoblock activity) and exposes the photo-sensitive paper. After about five minutes, participants squirt water on the paper to develop the image, resulting in interesting and beautiful two-dimensional representations of the objects inside the microscope.
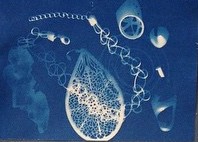
This is a nice analogy for TEM: with a beam of electrons instead of UV light, and nano-scale materials instead of pompoms and jewelry, we get similarly monochromatic two-dimensional images that we have to interpret in order to understand what we’re looking at.

The TEM model can even demonstrate some interesting features of the real thing, like diffraction effects and distortions. And it did a great job of getting our participants enthusiastic about chemistry, nanotechnology, and “living room microscopy”!

REFERENCES
- Bennett, J. et al. First-principles and thermodynamics study of compositionally tuned complex metal oxides: Cation release from the (001) surface of Mn-rich lithium nickel manganese cobalt oxide. Inorganic Chemistry, 2018, 57(21) 13300-13311. DOI: 10.1021/acs.inorgchem.8b01855
- Mulchandani, A. et al. “Nanoblocks”: A Playful Method To Learn about Nanotechnology-Enabled Water and Air Treatment. Journal of Chemical Education, 2019, 96(4) 708-713. DOI: 10.1021/acs.jchemed.8b00535
- Hudson-Smith et al. A Macroscale Model for Hands-On Activities Demonstrating Transmission Electron Microscopy. Journal of Chemical Education, 2019, 96(7) 1377-1382. DOI: 10.1021/acs.inorgchem.8b01855

Thank you so much for bringing real science into the Sandbox (our activity space)!
oh the video is so cute: https://m.facebook.com/groups/2204992216?view=permalink&id=10156839861027217&sfnsn=xmmo