Did you know that 2019 is the International Year of the Periodic Table? We chemists in the Center for Sustainable Nanotechnology are happy to celebrate this all year long!

The periodic table as we know it was first published 150 years ago. Its structure is familiar to anyone who has taken a chemistry class in high school or college: the 100 or so elements that make up the universe are arranged in rows according to their atomic number, and in columns according to their properties.

The atomic number of an element corresponds to how many protons it has in its nucleus. For example, carbon (C ) is number 6 in the Table; that means all carbon atoms in the universe have exactly 6 protons in their nuclei. If we pick a column in the periodic table, we can find that the elements in that column have similar properties. The last column on the right, for instance, contains helium (He), neon (Ne), argon, (Ar), krypton (Kr), xenon (Xe) and radon (Rn). All of these elements are gases at room temperature and pressure, and are very unreactive; hence, the common name for this column of elements is the “noble gases.”
Why the Periodic Table is Amazing
Organizing the periodic table by properties like this may seem obvious, but one of the triumphs of the table is that it relates these easily measurable properties (like boiling points, melting points, reactivity) to atomic-level properties. Mendeleev, the author of the periodic table as we know it, knew many chemical and physical properties of the elements, and even predicted that certain elements should exist before they were discovered (germanium, gallium).1 In order to appreciate how amazing this is, we need to understand a little bit about how atomic structure works.
Every atom contains a nucleus made of protons and neutrons. Each proton has a positive charge and a certain mass (1.67 x 10-27 kg to be exact). Neutrons also have a certain mass (almost but not quite the same as the proton), but no charge. Finally, far from the nucleus, are electrons, each of which has a negative charge and a certain mass (9.109 x 10-31 kg). If the positive and negative charges of protons and neutrons balance each other out, the atom will have a neutral (or zero) charge. Going back to our example of carbon, a neutral carbon atom will always have 6 protons in the nucleus and 6 electrons outside the nucleus. In addition, there can be either 6, 7, or 8 neutrons in the nucleus – we then describe the isotope of carbon based on the number of protons plus neutrons in the nucleus, giving us carbon 12, carbon 13, and carbon 14.

The 6 electrons in each carbon atom do not all have the same energy. You might remember that electrons in atoms are quantized in energy: they famously occupy the 1s, 2s, and 2p orbitals, written out as 1s22s22p2.
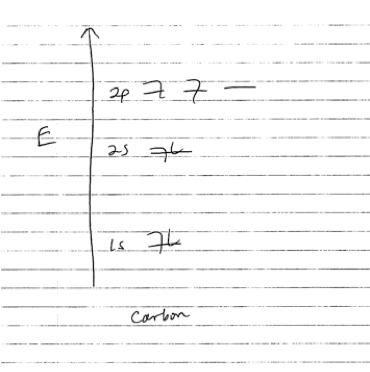
For more on energy levels and orbitals, see my earlier post about electrocuting pickles. For today’s purposes, what you need to know is that the 4 electrons in the top 2s/2p orbitals are called the atom’s “valence electrons.” These are the electrons that engage in bonding with other atoms nearby. If you have taken a chemistry class, you might remember that whenever you draw structures with carbon, you always show 4 bonds to carbon, using these 4 valence electrons.

All the other elements in the column of the periodic table below carbon (silicon, germanium, tin, lead) also have 4 valence electrons (although those electrons are in different orbitals) and they too make stable compounds with 4 bonds to other elements. Germanium was not known in Mendeleev’s time, but he knew of the others, and therefore predicted that germanium ought to exist, have the atomic number we know now, and the reactivity we know now!
Coming back to the periodic table, the amazing thing is that 150 years ago, no one knew electrons existed at all, let alone that they had particular energy levels! But the reactivity of the elements is dominated by their valence electron counts, so by organizing the elements according to their reactivity, Mendeleev anticipated this atomic feature that had not been discovered yet. It makes beautiful sense that elements with the same physical and chemical properties have the same number of valence electrons, and ended up organized together on the periodic table.
A Periodic Table of Nanoparticles?
Nanotechnologists have tried to come up with a “periodic table of nanoparticles” – here is one simplified example:
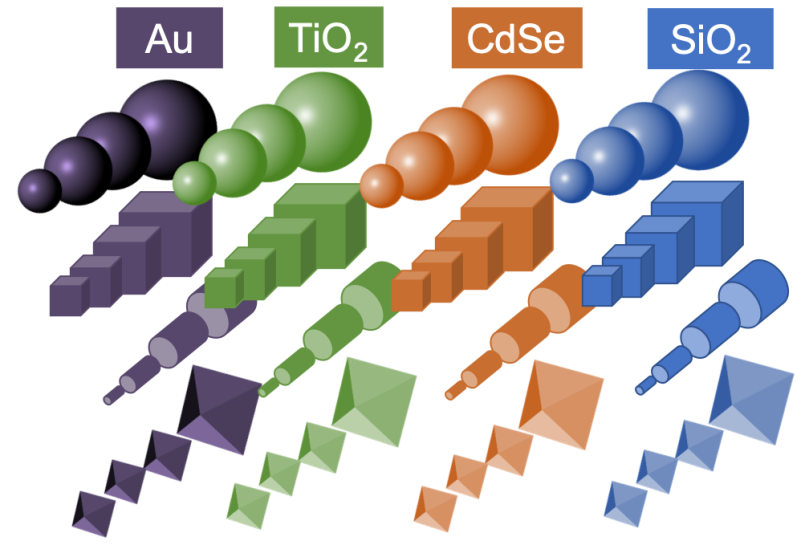
The idea is that one could organize nanoparticles on a table according to their special properties, and use that organizational system to create larger structures in a rational way, just like atoms are bonded together to create molecules. This example includes information about nanoparticle sizes and shapes, and so their “periodic table” is three-dimensional: composition (what the particle is made of) is along one axis, size another, and shape another.
Here’s another example that is less like the Periodic Table we’re used to seeing:

Regardless of how a “periodic table” of nanoparticles would be laid out, there are some big differences between individual atoms and individual nanoparticles:
- Size. Individual atoms are about 1x 10-10 m in diameter, but nanoparticles are usually 10 to 1000 times larger. Of course, nanoparticles are made of atoms!
- Heterogeneity. Atoms in a sample of a pure element are chemically identical, but individual nanoparticles in a batch can vary a little bit in size, shape, and surface characteristics.
- Shape. All atoms are approximately shaped like a sphere, with a very tiny nucleus (diameter 1×10-15 m) enrobed in a cloud of electrons (overall diameter 1×10-10 m). But nanoparticles can be made into spheres, rods, stars, and many other shapes.
The heterogeneity of nanoparticles means it is hard to get them to assemble into well-defined structures. For instance, you could try to put molecules on the surface of a nanoparticle so it would make a defined number of “bonds” to other nanoparticles:
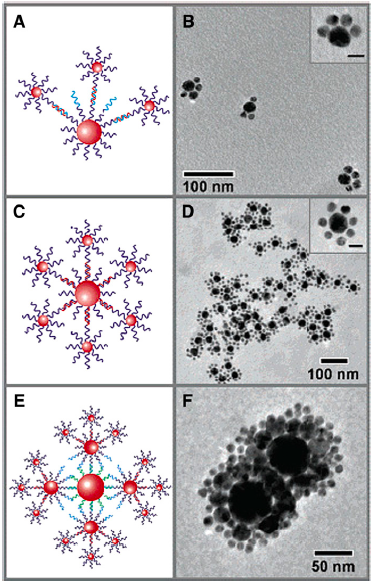
But as you can see from the microscopy images above, nanoparticle-nanoparticle “bonds” produce rather messy-looking materials. Even if you tried to arrange nanoparticles on a surface in a well-defined way, the reality is never as nice as the imagined structure.
If we could organize nanoparticles in a periodic table like atoms, we would be able to predict what types of new nano-based materials we could make, and what their properties might be. Chemists love making new compounds, and having guidelines is always helpful on the road to discovery. In the end, Nature is the best chemist of all!
We in the CSN salute the periodic table of the elements and continue to find inspiration in its form and structure!

REFERENCES
- Kean, S. Blogging the Periodic Table. Slate, July 20, 2010.
- Packwood, D. & Hitosugi, T. Materials informatics for self-assembly of functionalized organic precursors on metal surfaces. Nature Communications, 2018, 9, article number 2469. DOI: 10.1038/s41467-018-04940-z
- Xu, X. et al. Asymmetric Functionalization of Gold Nanoparticles with Oligonucleotides. JACS, 2006, 128, 9286-9287. DOI: 10.1021/ja061980b

