Last month, I went on a dream trip – a 9-day tour through Morocco. Morocco is a beautiful country at the cultural crossroads of Africa and the Middle East, with a touch of Europe (it was a French colony for much of the 20thCentury). We visited big cities like Casablanca, Fez, and Marrakech, but my favorite parts of the tour were when we visited the dunes in the Sahara Desert and villages in the Atlas Mountains. It was in one of these mountain villages, specifically Aït Ben Haddou, that I found an exciting example of sustainable chemistry.

Our tour guide introduced us to a local artisan who was making beautiful paintings of the desert and mountain landscape using only natural pigments. For the yellow of the sand, he mixed saffron threads in water. For the blue of the sky, he used indigo in water. For the brown/black skin color, he mixed green tea and sugar in water. These substances are more sustainable than manufactured paints and pigments because artists can use them basically as they are when they grow – almost no extra materials or processes are needed.

I could see the yellow and blue as he applied it to paper, but couldn’t really see the brown/black color. After he finished painting, he turned on a torch and gently heated the paper – the brown/black brushstrokes bloomed on the page. The simple pictures he painted were beautiful, and I bought one to remind me about the natural science and art present in Morocco.

When I returned from the trip, I did some follow-up reading to understand the chemistry behind these natural pigments. The molecule responsible for the bright yellow color of saffron is called crocin, and its molecular structure looks like this:
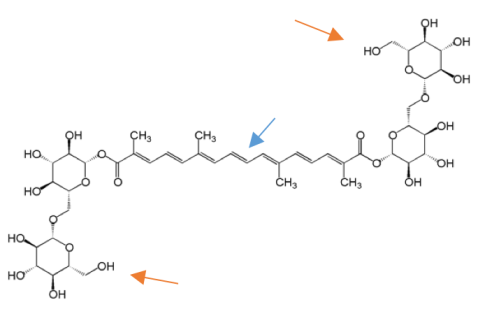
In addition to the O (oxygen) and H (hydrogen) atoms shown in the diagram, at every corner where two or more lines intersect there is a C (carbon) atom. Two lines very close together in the structure (as indicated by the blue arrow) represent what chemists call a “double bond.” What this means is that there are some extra electrons in the area between two carbon atoms. When a molecule has a lot of double bonds, it usually means that it can absorb some wavelengths of visible light and reflect other wavelengths. This is important because it means that our eyes perceive color based on the reflected light, rather than the substance just looking white or clear. (For more on light and color, see our previous blog posts here, here, and here.)
In addition to its bright yellow color and double bond molecular structure, another important thing about crocin is that it has two sugar molecules stuck on to the ends of its structure (as indicated by the orange arrows). This makes the molecule water soluble, which is why the artisan I met can just put saffron in water and get a nice pigment.
The molecule responsible for the blue color of indigo is this one:
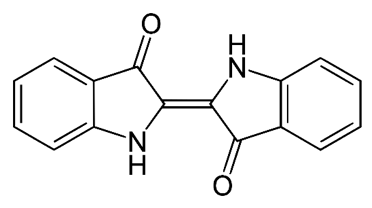
You’ll notice that this molecule also has a lot of double bonds, so it absorbs visible wavelengths of light such that our eyes perceive a blue color. Indigo is a relatively small molecule (meaning there aren’t many atoms needed to make up the structure), and the presence of oxygens (O), nitrogens (N), and hydrogens (H) makes it soluble in water so that it can also be used as a pigment. In addition to paint, you are probably familiar with indigo as a pigment in blue jeans!
The chemistry behind the final brown/black color is slightly more complicated. Green tea contains a lot of different molecules, and these specific molecules vary based on the type of green tea used. I did not quiz the Moroccan artisan about his sources of green tea, so I can only make some educated guesses about the chemistry behind this pigment. It seems likely that one critical component of the eventual brown/black color is a molecule known as catechin:
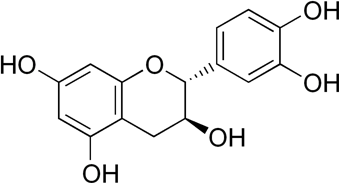
This molecule alone is not colored, but when it is stabilized (by the addition of sugar) and then heated (with the torch), the small catechin molecule will polymerize. This means that several or many of the individual molecular structures pictured above will chemically link together; the example structure below, three catechin molecules are linked together.

When this happens, all those double bonds end up near each other so the molecule absorbs light, and we see color. This means of course that the artisan I met could control how brown or black the pigment ended up by controlling how he applies heat to the paper (because that controlled the extent of polymerization).
Overall, I love the idea of using naturally occurring, sustainable materials wherever we can. Ancient civilizations clearly knew how to do that, and I really enjoyed seeing how a modern day artist used chemistry to make art that I can hang in my office back in Minnesota to remind me of my travels.

EDUCATIONAL RESOURCES
- Blatti, J. Colorful and Creative Chemistry: Making Simple Sustainable Paints with Natural Pigments and Binders. Journal of Chemical Education, 2017, 94 (2), pp 211–215 doi: 10.1021/acs.jchemed.6b00591
- Royal Society of Chemistry: Making Paint with Minerals lesson plan
REFERENCES
- Ncube, B. & Van Staden, J. Tilting Plant Metabolism for Improved Metabolite Biosynthesis and Enhanced Human Benefit. Molecules, 2015, 20(7), 12698-12731. Doi: 3390/molecules200712698
