Remember all those great Black Friday deals on QLED televisions? You may not realize it, but they were all about nanotechnology! The Q in QLED stands for quantum dots, which are not only being used to enhance the displays of TVs, but also are used in solar cells, medical imaging, and sensing.1-3 However, the disposal of these particles is not well regulated, leading to concern over their release into the environment. In the frenzy of holiday shopping, have you ever stopped to wonder what could happen if a quantum dot lands on the surface of a cell?

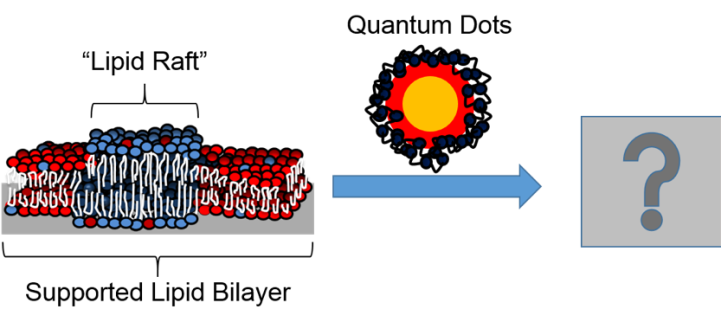
As an analytical chemist, my mind is constantly blown by the suite of analytical tools that we have in the Center for Sustainable Nanotechnology to study really hard scientific questions like this one. I recently used two of these analytical tools, the atomic force microscope (AFM) and the quartz crystal microbalance (QCM) to tackle the tricky question about quantum dots on a cell surface. In our study, we looked at how quantum dots interact with supported lipid bilayers, which (as we explained in a previous blog post) we can use as a mimic of the cell membrane. The paper was called “Quaternary Amine-Terminated Quantum Dots Induce Structural Changes to Supported Lipid Bilayers.” 4

Let me break down why this problem is so tricky and why it required really fancy tools to be able to study it. Everything we were studying was too small to be seen by eye or even using regular microscopes – the nanoparticles were about 6 nm and the lipid bilayers were about 4-5 nm – so we needed to use tools that allowed us to really zoom in to the nanoscale to get an idea of what was happening. Furthermore, the cell membrane of an organism is naturally wet, so we needed tools to allow us to work in liquids. Finally, the interactions between nanoparticles and membranes are dynamic, meaning they can change from moment to moment, so we really wanted to use tools that allowed us to monitor the interactions of the quantum dots and bilayers over time and not just take a single snapshot.

With these requirements in mind, I set out to design a system that we could use to understand these interactions in liquid and in real time. We chose to work with supported lipid bilayers that contain something called phase-segregated domains, or “lipid rafts.” These lipid rafts are found in the cell membranes of different organisms, from plants to animals to bacteria, and are important for moving things in and out of the cell, which makes them very interesting to study. Furthermore, my collaborator, Dr. Eric Melby, previously showed that 4-nm, positively charged gold nanoparticles attached more to supported lipid bilayers that had lipid rafts than those that didn’t (you can read more about his work here). This suggested that lipid rafts may play an important role in nanoparticle interactions with cell membranes, which was something I wanted to explore further with different types of nanoparticles, namely quantum dots.
To start, I used Eric’s method of forming supported lipid bilayers either with or without lipid rafts using quartz crystal microbalance. As I’ve described previously, QCM is a very sensitive balance that uses a quartz crystal to measure changes in frequency, which we can use to figure out changes in mass. For example, if we add quantum dots to a bilayer formed on the quartz crystal and notice that the frequency starts to decrease, this tells us that the bilayer is getting heavier because the added quantum dots are sticking to it.
In my experiments, we saw that when we added quantum dots to bilayers with or without lipid rafts the frequency decreased over time (Figure 5). This told us that the quantum dots were attaching to our bilayers. Interestingly, when we rinsed the bilayer with buffer (to get rid of any loosely attached quantum dots), we first saw a decrease of mass (likely due to quantum dots leaving the bilayer) and then saw another increase in mass before the measurement leveled off. This was the first time that we had observed this type of change using QCM before. We hypothesized that this was due to the quantum dots causing some sort of restructuring of the bilayer, such as holes, multilayers, or a combination of events. But with QCM alone, we were unable to say for certain what was happening.

Because we were uncertain what impact the quantum dots were having on the structure of the bilayer, we decided to use another analytical technique to get an actual picture of what was happening. This time we used atomic force microscopy (AFM). This technique allows us to study these interactions in liquid and over time, which if you remember were two very key factors to this work. I’ve described AFM in detail previously here, but briefly AFM works by using a very sharp tip that is attached at the end of a cantilever. We line up a laser to the end of this tip, which reflects off the tip onto a sensitive detector. As the tip scans across the sample, the laser light will move up or down on the detector depending on the height of the sample. From these changes in the laser’s position, we’re able to determine how tall features of the sample are.
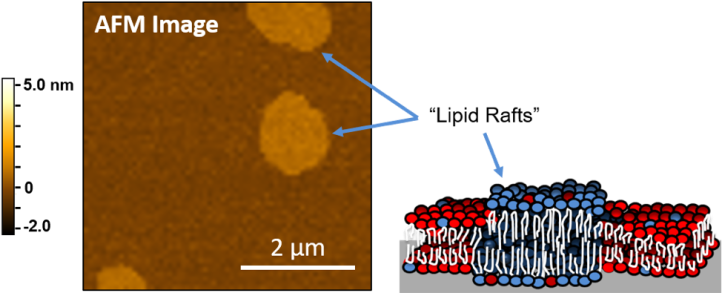
For the first part of our AFM experiment, we formed lipid bilayers with lipid rafts. These rafts are about 1 nm taller than the other part of the bilayer. You can see this in Figure 7, where the brighter regions of the bilayer are the lipid rafts.
To investigate the impact of quantum dots on these bilayers, we added quantum dots to the bilayers and collected AFM images over time. This allowed us to monitor the changes to the structure of the bilayers. Figure 8 shows what we found – and it was pretty neat!
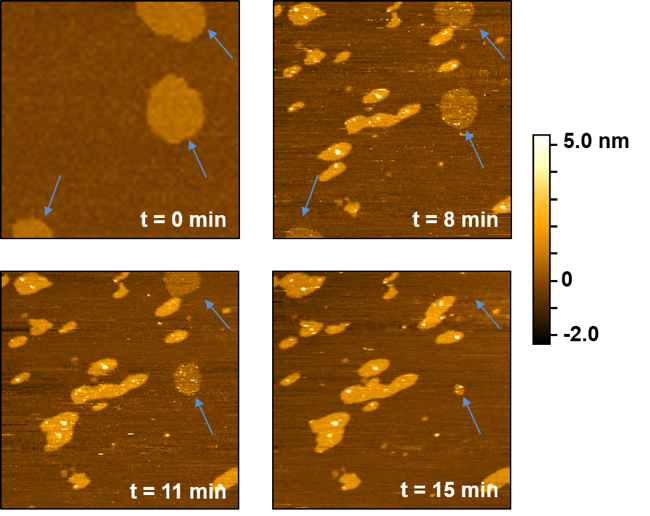
When we added quantum dots to the lipid bilayers, the lipid rafts shrank and eventually disappeared! It only took about 15 minutes for them to completely disappear. You can see this by following the blue arrows in Figure 8. The other bright regions in the images are quantum dots binding to the bilayers and inducing structural changes (increasing the height in these regions or burrowing into the bilayer). These two changes are consistent with the mass changes we saw using QCM. We believe that the lipid rafts collapse because of an increase in energy due to the addition of the quantum dots. Basically, it is easier for the lipid rafts to mix together with the other components in the bilayer rather than stay separated.
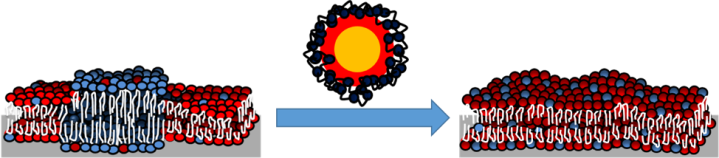
So, you might be wondering what all of this means. Well, to summarize, we found that positively charged quantum dots attach to supported lipid bilayers either with or without lipid rafts present. They also cause restructuring of the bilayers. In particular, when lipid rafts were present, the quantum dots actually caused the collapse of these important cell membrane components. Lipid rafts are found in the cell membranes of many different organisms, so this could have important implications in figuring out how nanoparticles affect different organisms.
But like with all good studies, there are still many more questions to explore! For this study we used supported lipid bilayers, but it would be really interesting to look at lipid rafts naturally within the cell membranes of actual organisms to see if we see the same effects. Furthermore, we can consider different types of nanoparticles with different surface coatings and see if that changes the results. So, the next time I see a QLED TV at the store, I’ll be sure to admire its beautiful colors, but I’ll also be thinking about my next research project.
ADDITIONAL RESOURCES
- Quantum dots go on display: Adoption by TV makers could expand the market for light-emitting nanocrystals. by Katherine Bourzac in Nature, 2013.
- What are quantum dots? in Nanowerk
REFERENCES
- Martynenko, I. V.; Litvin, A. P.; Purcell-Milton, F.; Baranov, A. V.; Fedorov, A. V.; Gun’ko, Y. K. Application of semiconductor quantum dots in bioimaging and biosensing. Materials Chemistry B, 2017, 5, 6701−6727. doi: 10.1039/C7TB01425B
- Rühle, S.; Shalom, M.; Zaban, A. Quantum-dot-sensitized solar cells. ChemPhysChem, 2010, 11, 2290−304. doi: 10.1002/cphc.201000069
- Medintz, I. L.; Uyeda, H. T.; Goldman, E. R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nature Materials, 2005, 4, 436-446. doi: 10.1038/nmat1390
- Mensch, A.C., Buchman, J.T., Haynes, C.L., Pedersen, J.A., Hamers, R.J. Quaternary amine-terminated quantum dots induce structural changes to supported lipid bilayers. Langmuir, 2018, 34, 12369-12378. DOI: 10.1021/acs.langmuir.8b02047

[…] to enhance the displays of TVs, but also are used in solar cells, medical imaging, and sensing.1-3 However, the disposal of these particles is not well regulated, leading to concern over their […]
[…] to enhance the displays of TVs, but also are used in solar cells, medical imaging, and sensing.1-3 However, the disposal of these particles is not well regulated, leading to concern over their […]