A lot is being said these days about pollution in waterways, how to prevent it, and how to clean up pollution that started decades ago. During a summer spent doing scientific research here in Baltimore, I was able to see the effects of pollution on the Chesapeake Bay firsthand. I visited the Inner Harbor in Baltimore several times with my friends throughout the summer — and each time, the water was a dark green color. Murky and a little smelly at times, there was no doubt that the harbor was not very healthy.

Spanning 6 states and covering over 64,000 square miles, the Chesapeake Bay has always been important for the wildlife that it supports and the food that it provides to locals.1 In the early 1800s, oyster farming activities in the Bay became a crucial part of its economic identity.2 The economy was reshaped as manufacturing advances from World War II paved the way for cheap and effective chemical fertilizers, so crop and animal agriculture became the new leading economic drivers. Fast-forward several decades and now agricultural pollution, or pollution resulting from the activities of farmers and produce growers, is also one of the leading sources of pollutants that make their way into the Bay, contributing more than 50% of the total sediment pollution there.3

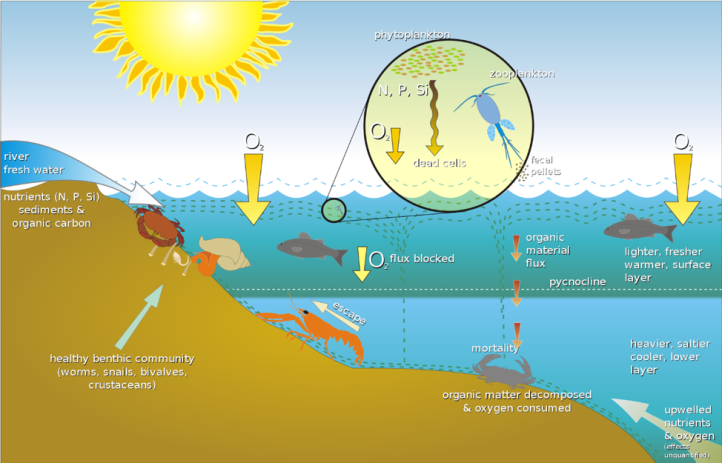
So, what is agricultural pollution and what is its impact on the environment? When we think about pollution, most people tend to imagine harmful gases leaking into the air or tanks marked with skull and crossbones dumping hazardous chemicals into rivers and lakes. But agricultural pollution is actually the leaching of nutrients necessary for plant growth into waterways. The main culprits of this kind of pollution are nitrogen and phosphorus, which are found in both chemical fertilizers and manure. When it rains, the nutrients from these fertilizers can leach into the ground and flow into the Bay through a process called runoff. Even when there is no rain, nutrients that seep into the groundwater below can make their way into the Bay in a similar manner.4

The Eastern Shore of Maryland is home to a very robust poultry industry and has been a key source of agricultural pollutants leaching into the Chesapeake Bay.3 As a result, the area surrounding that part of the Bay receives more than twice the amount of nitrogen and phosphorus compared to the rest of the Bay. Moreover, estimates made by the United States Geological Survey suggest that more than 90% (by mass) of that pollution is a result of fertilizer applications.5
Since fertilizers are relatively inexpensive, easy to use, and can greatly benefit their crops, farmers apply generous amounts of them each growing season. The Maryland Department of Agriculture estimates that each year, about 228,000 tons of extra chicken manure is being applied beyond what would be needed to effectively fertilize the state’s crops.6 One reason pollution from fertilizers is a problem is that the migration and transport of excess nutrients through processes such as runoff can cause massive algal blooms. These huge growths of algae can suffocate the wildlife around them and create “deadzones,” in which no living organism can survive.5 (There is a toxic algal bloom afflicting the Florida Coast right now.)
Even more concerning, perhaps, are the hazards that agricultural pollution in the Chesapeake Bay poses to public health. As a consequence of agricultural pollution, not only are animals both on land and in the water dying, but their decomposing organic matter also releases chemicals that are dangerous to people who might want to swim or play in the Bay.
Luckily for us, both the state and federal governments have taken actions and set goals to reduce pollution in the Bay, and althought progress has been slow, it is promising. Most recently, a 2012 Chesapeake Bay Commission assessed the state of the Bay and estimated that it would cost $14 billion to restore the Eastern Shore to its former glory by 2025, but this goal is unlikely to be met based on the current trajectory.1 There have been other efforts to promote Best Management Practices among farmers to reduce fertilizer use and instead implement inexpensive alternatives1, as well as several plans intended to divide up the cost of pollution management (for more on this, see the Additional Resources section below).
To go along with current land and pollution management practices (which can be limited if only a few farmers adopt them), researchers are turning their attention towards nanoscience and nanotechnology as a way to combat agricultural pollution. Only a few nanotechnology-related projects have been proposed to combat agricultural pollution specifically in the Bay so far, but one that has been gaining some attention is the use of a nano-magnetite nanocomposite for phosphate removal through magnetic fields. Essentially, while bulk magnetite (a rock mineral and common iron ore) produces a very weak magnetic field, the nano-magnetite particles are supermagnetic, which allows them to attract free phosphates in solution. This property also allows for easy removal of the nanocomposite from solution using magnetic fields.7 This is not the only example of nanotechnology’s utility in helping to combat agricultural pollution. Some other potential areas of nanotechnology application include the use of carbon nanotubes for water filtration, coating seeds with nanoparticles that can help speed up germination, and nano-enabled enhanced delivery of nutrients through either the soil or through sprays.8

Although the application of nanotechnology to agricultural practices has great potential for helping to reduce agricultural pollution, it still is a relatively new field of interest and there are some risks to investigate. A previous blog post explained how nanoparticles can potentially leach into the environment and potential outcomes of this exposure. Before farmers start widely using nanomaterials in agriculture, researchers need to do more research, including working to understand the toxicity of nanoparticles to both the environment and to humans. In addition, international and domestic regulation of nanoparticle use is evolving and will affect how these new agricultural technologies will be implemented.8
While the Bay is facing tough times in regard to agricultural pollution, all is not lost, and many organizations are constantly striving for a successful restoration of the Bay’s health. Nanotechnology can help reduce the human impact on the Chesapeake Bay. As with many emerging fields, there are certainly potential risks to go along with the rewards, but there is still hope! While progress may be slow, agricultural pollution is fortunately reversible and there are plenty of options left to explore.
ADDITIONAL RESOURCES
- The Chesapeake Bay and Agricultural Pollution: The Problem, Possible Solutions,
and the Need for Verification - Restoring the Health of the Chesapeake Bay
- Understanding Nutrients in the Chesapeake Bay Watershed and Implications for Management and Restoration – the Eastern Shore
- Examining Nanotechnology for Recovery of Phosphorus
REFERENCES
- Kobell, Horton, Simpson, & Summers. The Chesapeake Bay and Agricultural Pollution. Abell Foundation, 2015. Retrieved from https://www.abell.org/sites/default/files/publications/env-agrunoff1215.pdf
- Chesapeake Bay, Our History and Our Future. The Mariner’s Museum, 2002. Retrieved from https://www.marinersmuseum.org/sites/micro/cbhf/home.html
- How Farms Affect the Chesapeake Bay’s Watershed. The Earth Observatory, 2016. Retrieved from https://earthobservatory.nasa.gov/images/88523/how-farms-affect-the-chesapeake-bays-water
- Davies, O’Brien, & Reed. Impacts of Lawn Fertilizer. Vermont Legislative Research Shop, 2001. Retrieved from http://www.uvm.edu/~vlrs/doc/lawnfert.htm
-
Ator, S.W., and Denver, J.M., 2015, Understanding nutrients in the Chesapeake Bay watershed and implications for management and restoration—the Eastern Shore (ver. 1.2, June 2015): U.S. Geological Survey Circular 1406, 72 p., doi:10.3133/cir1406.
- Baker, W. A manure solution for the Chesapeake Bay. The Washington Post, 2015. Retrieved from https://www.washingtonpost.com/opinions/a-manure-solution-for-the-chesapeake-bay/2015/01/09/5cc1ff26-8d57-11e4-8ff4-fb93129c9c8b_story.html
- Finlay, Kumar, Viswanathan, & Weinberg. Examining nanotechnology for recovery of phosphorus. Industrial WaterWorld, 2018. Retrieved from https://www.waterworld.com/articles/iww/print/volume-11/issue-4/feature-editorial/examining-nanotechnology-for-recovery-of-phosphorus.html
- Bakker, Brauers, van der Elst, & Wangari. Nanotechnology in Agricultural Production. UN Policy Analysis Branch, Division for Sustainable Development, 2016. Retrieved from https://sustainabledevelopment.un.org/content/documents/12872Policybrief_Agri.pdf
- Jiang, L., & Gao, L. (2003). Carbon Nanotubes−Magnetite Nanocomposites from Solvothermal Processes: Formation, Characterization, and Enhanced Electrical Properties. Chemistry of Materials,15(14), 2848-2853. doi:10.1021/cm030007q
