Almost 4 years ago now (wow I’m getting old) I wrote a blog post about the Invisible Remnants of Dead Stuff Hiding in the Water called natural organic matter, or NOM. Well, some colleagues and I recently published a new paper entitled “Natural organic matter concentration impacts the interaction of functionalized diamond nanoparticles with model and actual bacterial membranes,”1 in which we brought some of those initial experiments I discussed in my previous blog post to fruition and learned some really interesting things along the way. We set out to understand the impact that NOM can have on the properties of nanomaterials, and then subsequently how any NOM-induced changes to the nanomaterials may impact how they interact with bacteria. Let me explain what I mean:
NOM is a collection of breakdown products from once-living things (animals, plants, etc.) and their waste (feces, leaves, etc.). Because it is composed of organic matter, it is mainly made up of hydrogen, oxygen, and carbon, but don’t let that fool you – NOM is very complex. The exact molecular structure of NOM is unknown and depends on the environment that it comes from. NOM in some amount is found in every environment you can think of. In our studies we used NOM that we collected from the Suwannee River in Georgia, which is standard for NOM from the International Humic Substances Society (IHSS) (you can read more about this organization here). This particular river was chosen as a standard by the IHSS due to the high concentration of NOM in the river and the relatively low human impact in this area.

One goal for our study was to help understand how nanoparticles affect organisms in a river environment. So let’s think about two of the possibilities that can happen when a nanoparticle enters a river: it could either remain suspended in the water column, or it could sediment out to the bottom of the river. In both of these scenarios it could also be taken up (e.g. eaten) by organisms like fish or bacteria. However, since NOM is found ubiquitously in the environment, before we think about how nanoparticles might affect those organisms we really first need to think about how the nanoparticles could be impacted by NOM. It’s very likely that when a nanoparticle enters a river it will first encounter NOM. Therefore, we wanted to know what would happen to the properties of the nanoparticle when that happens. We focused on the impact on the size and charge of the nanoparticles because these two factors can affect how a nanoparticle is transported in an environment and where it ultimately ends up (suspended in the water column or sedimented out of solution).
To study this question, my colleague Marco Torelli (a grad student at UW Madison at the time) wrapped positively charged ligands called polyallylamine hydrochloride (PAH) around the outside of diamond nanoparticles. While diamond nanoparticles are unlikely to find their way into the Suwannee River (or any other river for that matter), members of the CSN are really good at controlling the size and surface chemistry of diamond nanoparticles. Having this controlled system helped us start to understand how NOM might impact the size and charge of the nanoparticles.

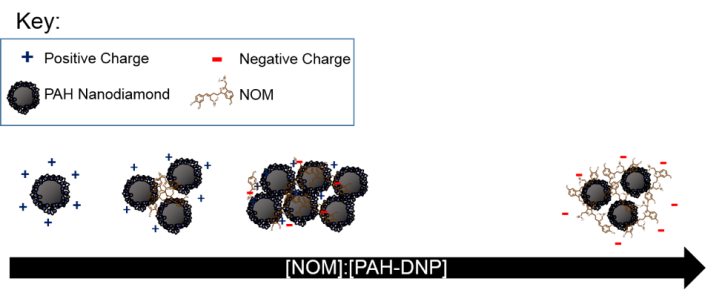
What we found was that NOM, which is negatively charged, was attracted to the positively charged particles. When we added only a little bit of NOM, we found that the overall charge remained nearly the same (positive), but the particles got a little bit bigger in size. This means that the NOM caused the particles to aggregate, or clump together. As we added more and more NOM, the particles still clumped together but became more negatively charged. You can see this summarized in Figure 2. Ultimately, we found that the amount of NOM present plays a big role in the size and charge of the diamond nanoparticles. If these particles were released into the environment, their size and charge likely won’t stay the same as when they started, which is a really big consideration when thinking about how a nanoparticle behaves in the environment.
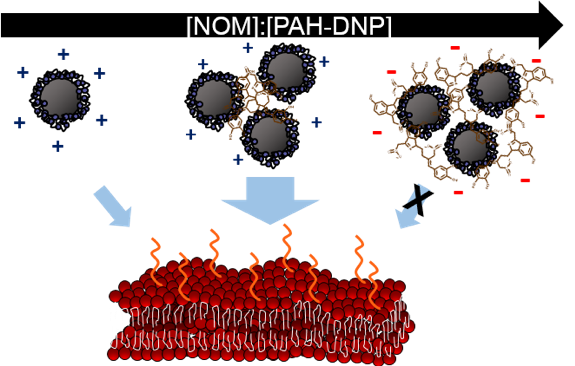
Realizing that NOM can change the properties of the diamond nanoparticles, we next wanted to explore how these changes in properties could impact nanoparticle interactions with organisms. We focused on bacteria as a model organism and thought about what would happen when a nanoparticle first encountered a bacterium. Likely, the nanoparticle would first hit the bacteria’s outer cell membrane. Therefore, we decided to study how diamond nanoparticles attached to supported lipid bilayers (a model for cell membranes). To make these membranes “look” as similar to bacteria as we could in the lab, we included lipopolysaccharides (LPS), which are an important molecule found in the outer membrane of Gram-negative bacteria, into some of the model membranes we studied. (For more on what LPS has to do with nanoparticles sticking to bacteria, see this post).
We used quartz crystal microbalance with dissipation monitoring (which you can read more about here) to see how much attachment there was between diamond nanoparticles and lipid bilayers in the presence and absence of NOM. We found that small amounts of NOM (when the particles were aggregated, but still positively charged) increased attachment of diamond nanoparticles to bilayers, whereas high amounts of NOM (when the particles were aggregated and negatively charged) eliminated attachment to bilayers. This means that not only does NOM have the ability to change the properties of the diamond nanoparticles, it can also impact the interactions with the cell membranes of bacteria.
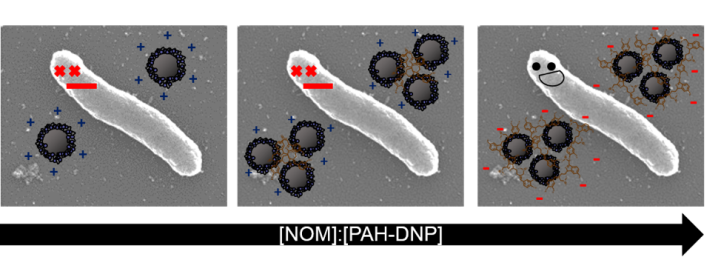
To extend these studies to full bacteria, my collaborators at Augsburg University, Rodrigo Tapia Hernandez and Josh Keuther, conducted similar studies looking at the interaction between the diamond nanoparticles and a Gram-negative bacteria, Shewanella oniedensis. They noticed similar trends, where low amounts of NOM caused the particles to be toxic to the bacteria, but high amounts of NOM eliminated the toxicity. To dig even deeper, they did a test where they looked at the amount of membrane damage that was induced by the diamond nanoparticles in the presence and absence of NOM. They saw that high concentrations of NOM eliminated membrane damage, which was consistent with our supported lipid bilayer work where we saw no attachment of particles at high NOM concentrations. It was really fun to see how we could correlate results on a model membrane system to actual bacteria!
Our results highlight the importance of considering natural organic matter when thinking about the impact of nanoparticles on different organisms in the environment. We showed how NOM can impact the properties of nanoparticles and also subsequent interactions with organisms. Ultimately, NOM may work in our favor to make nanoparticles less toxic, but so far we’ve only shown that for one particular nanoparticle and one type of bacteria so there’s still a lot of work left to be done!
REFERENCE
- Mensch, Arielle C., Rodrigo Tapia Hernandez, Joshua E. Kuether, Marco D. Torelli, Z. Vivian Feng, Robert J. Hamers, and Joel A. Pedersen. “Natural Organic Matter Concentration Impacts the Interaction of Functionalized Diamond Nanoparticles with Model and Actual Bacterial Membranes.” Environmental Science & Technology, 2017, 51, 19: 11075-11084. doi: 10.1021/acs.est.7b02823
