Engineered nanoparticles (NPs) are now components of thousands of consumer products, including personal care products, sporting goods, solar panels, and next-generation batteries. In fact, demand for electric car batteries (built using lithium-nickel-cobalt nanosheets) might cause lithium prices to skyrocket in the coming decade, and completely change the way in which the element lithium is recovered and processed. Engineered NPs pose a poorly-understood (but potentially meaningful) threat to human and environmental health, because they have unique size-dependent properties and reactivity, not found in molecular chemicals or bulk materials (like metals or semiconductors). As with any chemical, the risk that engineered nanomaterials pose to human or environmental health is related to both their hazards (possible toxicity) and their bioavailability (for more on risk vs. hazard, see this blog post).
But how do we know what happens to NPs when they get into the environment?

Bioavailability is a single concept unifying various important factors that determine how easily chemical contaminants might be taken up by organisms.2 For example, how quickly nanoparticles travel through ecosystems, whether their chemical composition changes in the environment, and whether organisms that encounter them will consume them, all contribute to bioavailability. The easier it is for nanoparticles to move around in soil or ground water, the more likely any nanomaterial contamination would be to become widespread. Alternatively, if nanomaterials are quickly trapped by algae, biofilms, or terrestrial plants, they might be ingested in large quantities, and quickly biomagnified up the food web. Biomagnification is a significant concern for accurately predicting human exposure to other contaminants, like heavy metals or persistent organic pollutants (you can read more about biomagnification in this blog post).
One of the Center for Sustainable Nanotechnology’s primary goals is to connect nanoparticle properties like size, shape, and composition to the way NPs interact with biological systems. A typical engineered nanoparticle consists of a metal, diamond, or semiconductor crystalline core, which is then coated with small organic molecules called “capping agents.” Typical capping agents include small molecules like citric acid (the molecule responsible for the sour taste in lemons), ascorbic acid (Vitamin C), or surfactants. You can learn more about the composition and synthesis of engineered NPs in this blog post. Designing effective experiments that relate NP properties to their bioavailability is more challenging than it first appears, though, because the size and composition of nanomaterials often change when they enter a new environment, be it groundwater, soil, or blood.
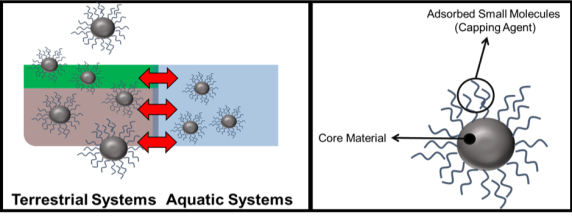
When nanoparticles are made in the research lab, their properties and composition can be precisely defined. For instance, the size of the nanoparticle’s core can be determined with an electron microscope. The coverage of the organic molecules on the particle’s surface can be determined using other chemical instrumentation, like thermogravimetric analysis or 1H-NMR spectroscopy.3 The size, composition, and type of molecules on the particle’s surface constitute a “synthetic identity” for the nanoparticle, similar to a finger print. As long as the nanoparticle stays in the research lab, dissolved in highly purified (deionized) water, this synthetic identity remains more or less intact. Once the NPs enter the real world (for example, maybe one day they will be injected into a patient’s blood stream), the NPs may experience many changes: they may stick to proteins present in blood, high salt concentrations may cause the particles to clump together, or the particles may break down into the individual metal ions or atoms that make up the core. As a result, the “biological identity” of a nanoparticle can be entirely different from their original “synthetic identity.”
Both these “physiochemical transformations” of the NPs and their odd size (somewhere between a typical protein and a very large virus), scientists predict that most engineered nanomaterials probably move through ecosystems quite differently than typical chemical contaminants, like TCE or PAHs. Many nanoparticles are similar in size to the kinds of particles and large molecules that make up soil, algae, and bacterial biofilms, while molecules like TCE and PAH are much smaller.
Our center recently published a research study in the journal ACS Sustainable Chemistry & Engineering that examined how engineered nanoparticles might move through soil. We set up several simple laboratory models to imitate soil, algae biofilms, and ground water. Then we introduced some nanomaterials to our simulated soil and biofilm matrices, and measured how they moved using some basic chemical instruments (such as absorbance spectroscopy).4

A final challenge in this kind of study is that there are so many different kinds of nanoparticles! We began this study by looking at gold nanoparticles (check out the image below). Why? Gold nanoparticles are particularly convenient engineered nanoparticles to study. They are easy to prepare in different shapes and sizes, easy to track through soil with simple laboratory instruments, and they are typically considered to be very stable. For instance, unlike iron, which rusts easily, gold doesn’t tarnish. And unlike silver, which quickly leaches silver ions into water, gold doesn’t dissolve easily.

Going into our experiments, we expected that the gold nanoparticles would remain intact in the soil and simulated ground water, and that the nanoparticles would move as individual particles, with their “synthetic identity” intact through our soil and algae columns. The ability to track the gold nanoparticles’ progress through our soil samples was key to a successful experiment – many nanoparticles made of non-precious metals cannot be easily detected, even in relatively simple environmental systems.
We encountered a surprising result almost immediately; we noticed that some of the gold nanoparticles we tested seemed decidedly unstable. When gold nanoparticles break down, they merge into larger gold crystals through a process called aggregation, and the change is very obvious, even to the naked eye. The vibrant red or brown color of a solution of intact gold nanoparticles gives way to an (ugly) grey-blue. Several of the gold nanoparticles that we tested aggregated almost immediately when they were placed in simulated ground water. We found that the stability of the particle depended not on its size and shape, but on the chemical connection between the capping agent and the crystal surface. For instance, gold nanoparticles coated with citric acid merge to form larger nanowire structures quite quickly, because calcium ions in the ground water break the citric acid molecules loose from the nanoparticle surface. Loss of the capping agent opened up surface sites where the individual gold crystals could merge and form irregular wire-shaped structures. In contrast, gold nanoparticles that were wrapped in polymers (like polyacrylate) were more likely to retain their synthetic identity in soil or ground water.

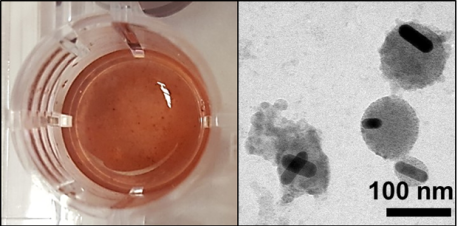
Another way that nanoparticles can change their synthetic identity when they enter soil or groundwater, is to become entangled in naturally occurring organic polymers (like alginic acid, an important building block of algae biofilms). We found that if alginic acid was present in our ground water samples, alginic acid polymer strands would stick to all the gold nanoparticles that we tested, winding the nanoparticles into large algae-nanoparticle capsules, which could actually be seen with the naked eye. The figure below shows both the large algae-NP capsules (the red-brown flecks visible in the left-hand picture) and electron microscope images of individual gold nanorods trapped in nanoscale polymer capsules. These algae polymer capsules change the effective size of the nanoparticles in ground water and may cause engineered NPs to move through the environment in the same way as clumps of algae.
Engineered NPs can change size, shape, or composition once they are released into the environment. These changes may include aggregating into larger crystals or becoming trapped in natural organic matter (such as algae) present in the environment. The nanoparticles’ new “biological identity” may make them less mobile in the environment, or more likely to “piggy back” along environmental transport pathways taken by algae, silicates, or aluminates.
What this means for future research is that, in some cases, it might be more important to understand whether these nanoparticle aggregates are toxic to bacteria and simple organisms, rather than whether the original “as synthesized” nanoparticles would be hazardous. Furthermore, it appears that the chemical connection between the capping agent and the nanoparticle crystal surface may be an important factor in determining whether the nanoparticle breaks down when it enters the environment, or whether the original NP remains intact. The Center for Sustainable Nanotechnology has already begun to consider the changeable nature of nanoparticles released into the environment, as we design new experiments to study their biological interactions.
Acknowledgement: We want to extend our thanks to a collaborator outside the CSN, Lee Newman, and another collaborator Jason White (who recently joined the CSN), who provided us with quality soil samples and excellent advice.
EDUCATIONAL RESOURCES
- Sci Show video on Biofilm: A New (Gross) Thing to Worry About
- TeachEngineering: Get in My Body: Drug Delivery lesson plan (grades 9-12)
- Science Friday: A DIY Groundwater Model (grades 9-12)
REFERENCES
- Yetisen et al. Nanotechnology in Textiles. ACS Nano, 2016, 10 (3), pp 3042–3068 DOI: 10.1021/acsnano.5b08176
- Semple, K. T. et al. Environmental Science & Technology, 2004, 38(12). DOI: 10.1021/es040548w
- Thermogravimetric Analysis (TGA): Frequently Asked Questions (accessed Feb 20, 2018).
- Lohse, S. E.; Abadeer, N. S.; Zoloty, M.; White, J. C.; Newman, L. A.; Murphy, C. J. Nanomaterial Probes in the Environment: Gold Nanoparticle Soil Retention and Environmental Stability as a Function of Surface Chemistry. ACS Sustainable Chemistry & Engineering 2017, 5(12), 11451–11458. DOI: 10.1021/acssuschemeng.7b02622
