Gold is one of the most highly desired metals in the world. The precious yellow metal is rare in nature and has been used as a medium of currency and in the making of jewelry since ancient times (Figure 1).1 Believed to be brought to Earth by meteors, gold is truly out of this world!2 There are a lot of reasons that gold is special and why it has maintained its value in our societies. But, gold might be even more special than we thought.

By crafting and processing this shiny metal, people have gradually learned its physical and chemical properties. “True gold fears no fire” is an old Chinese idiom, which originates from the fact that bulk gold stays intact and shiny when placed over wood fires (600-1000 °C), whereas most other metals (such as copper, zinc, and nickel and their alloys) would melt and/or tarnish in those conditions. In chemistry terms, we say that gold has excellent chemical stability against oxidation and a relatively high melting point (1064 °C). But it turns out that, thanks to nanoscience, there are ways to change how gold behaves at different temperatures while preserving its other special characteristics.
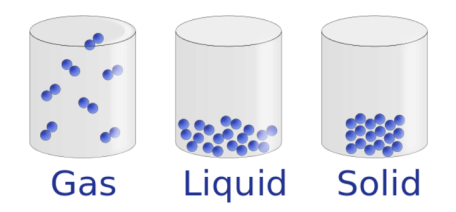
Let’s use our daily experience with water as an example to help understand what’s going on with gold. We know that ice (solid) seems to always melt to water (liquid) at a fixed temperature, and then water seems to always evaporate to steam (gas) at a fixed temperature. These temperatures are referred to as the melting point and boiling point, respectively. The melting and boiling points can be different depending on the local pressure. This is why your cooking or baking recipes might have different instructions for high altitudes, which have lower air pressure than low altitudes.
We don’t have to climb up and down mountains to manipulate the boiling point, though. We can easily do this by changing the local pressure, which is how a pressure cooker works: by increasing the pressure, the boiling point of water increases to a higher temperature, which means the water can get hotter without evaporating, which makes the food cook faster (Figure 2).
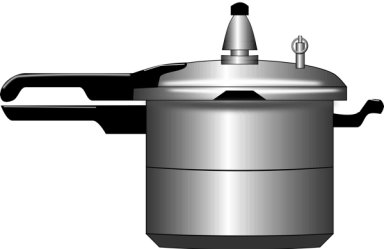
To understand how a pressure cooker raises the pressure inside, we have to remember what happens to the molecules of a liquid when it heats up: they start to move farther apart! Melting point, however, is much less sensitive to local pressure change. This is because compared to liquid evaporating to gas, the volume generally does not increase too much when solids melt into liquids. (Side note: water is unusual in that its volume actually gets bigger when it freezes to a solid – ever have a can of soda explode in the freezer? – but that’s a topic for another post!)
The main point it that it is not practical to manipulate the melting point of a substance by changing the local pressure – you would have to make a drastic change in pressure to get even a little change in melting point (Figure 3).
But, are there other ways to alter the melting point of a material? And getting back to the main topic of this post, can we melt gold at room temperature?
In an earlier blog post, “Nanoparticles Are All Around Us,” we discussed that as the size of a material decreases to nanoscale, many physical and chemical properties also change. This is mostly due to the “surface effect”, or the increased surface area-to-volume ratio (Figure 4).3

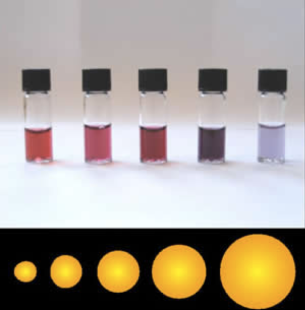
The color of gold, for example, changes from shiny yellow to dark red when its size decreases to the nanometer range (Figure 5). So how does the melting point of a material change as its size decreases to the nanoscale?
As early as 1871 (when he had no way of actually seeing nanoparticles), Sir William Thomson showed that the melting point changes inversely with the radius of a particle according to the following equation, known today as the Gibbs-Thomson equation4:
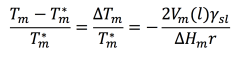 If you plug in information about the material’s particle size and other characteristics, this equation shows that a material’s size and melting point are directly related. As the size of the material decreases, the melting point will also decrease. This phenomenon is commonly known as “melting point depression”.5
If you plug in information about the material’s particle size and other characteristics, this equation shows that a material’s size and melting point are directly related. As the size of the material decreases, the melting point will also decrease. This phenomenon is commonly known as “melting point depression”.5

Figure 6 shows the relationship between nanoparticle size and melting point for gold according to the Gibbs-Thomson equation. As we can see, the melting point of gold nanoparticles can be even lower than room temperature (~23-25 °C) when the size decreases to less than about 1.4 nm. At that size, there are only about 85 atoms present in each nanoparticle, and most of the atoms are exposed on the surface.6 (In contrast, in a 4 nm particle, there are nearly 2000 gold atoms, leaving most atoms still on the inside of the particle. Wondering how we know this? See our blog post, “How can you calculate how many atoms are in a nanoparticle?“)
The difference between a solid and a liquid is easy to see for normal-sized objects: liquids move, flow, and take the shape of whatever containter that they are put in but solids are rigid and don’t slosh around.7 But is there a way to directly visualize the “liquid nanoparticles” we have been describing here?

Transmission electron microscopy (TEM) is very well suited for this purpose. When electron beams hit a sample, they can heat up and melt the nanoparticles. Atoms in a sample can also cause incident electron beams to diffract into many specific directions. By measuring the angles and intensities of these diffracted beams, diffraction patterns can be created and the position of the atoms in the sample can be determined. Atoms are generally highly ordered in solids but move around in liquids, which will result in different diffraction patterns. Spotty patterns are usually observed for solid samples whereas halo patterns are usually observed for liquid samples. We can then differentiate solid state from liquid state by looking at their electron diffraction patterns. (For more on electron microscopy, see our post “Nature Under a Microscope: Exploring the Beauty of Nanoscience”.)
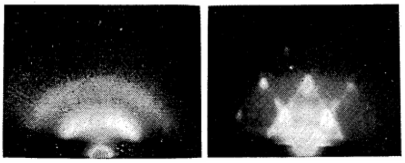
As early as 1954, Takagi first used this strategy to test out “melting point suppression” (Figure 8). He chose lead to study because it has a relatively low melting point, and can be easily made into a 5 nm-thick film. Under TEM, Takagi and his team saw that the melting point of a 5 nm-thick lead film decreased from the usual 327 °C to 170 °C.9
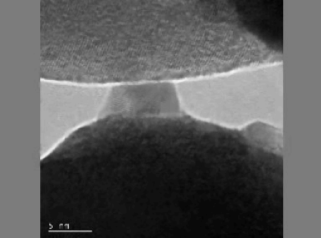
Although Takagi’s results were impressive, they were not able to capture the solid-to-liquid transition in real time. Nowadays we can do this thanks to the development of what is called in situ TEM.10 When silver nanoparticles smaller than 10 nm were placed on a tungsten tip, the researchers observed a “liquid-like” behavior under certain circumstances. The silver nanoparticles stayed highly crystalline in the interior, suggesting that they did not melt — if the particles had indeed melted, we would expect to see no crystalline patterns and a completely randomized atomic arrangement (like the halo pattern from figure 8). This interesting “liquid-like” behavior was attributed to the atoms on the silver nanoparticle moving around under pressure, giving the illusion that it’s melted (Figure 9).
All of this helps us understand that, even though people used to think that “true gold fears no fire,” we now know that nanoscale metals, including gold, can behave like liquids at room temperature!
On one hand, melting point depression could make some nanoparticles less useful, if they need to be in a solid state to function in their technological applications. On the other hand, melting point depression is also very helpful for applications where nanoparticles work better in liquid state. For example, we can easily change the shape of nanoscaled materials at much lower temperatures than their melting point would suggest.
Therefore, the answer to the question we started with is: Yes! In theory, gold, or any other material, can be considered as “melted” thanks to the amazing properties of materials at the nanoscale.
EDUCATIONAL RESOURCES
- The International Association for the Properties of Water and Steam: Why does water expand when it freezes?
- Journal of Chemical Education: The Science of Chocolate: Interactive Activities on Phase Transitions, Emulsification, and Nucleation by Amy Rowat et al. (may require subscription)
- University of Georgia Extension: The Science Behind our Food – Using Freezing-Point Depression to Find Molecular Weight
REFERENCES
- Krasimirov, A. The World’s Oldest Gold Artifact May Have Just Been Discovered. Huffington Post, Aug 10, 2016.
- Choi, C. Q. Asteroids May Have Brought Precious Metals To Earth. Live Science.com Sep 7, 2011.
- Mott, S. M. The Size Of Matter: Why Properties Change At The Nanoscale. Yale National Initiative.
- Thomson, W. On The Equilibrium Of Vapour At A Curved Surface Of Liquid.
The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science. 1871, 42, 448. - Lu, H. M., et al. Size-, Shape-, And Dimensionality-Dependent Melting Temperatures Of Nanocrystals. Physical Chemistry C. 2009, 113, 7598-7602.
- Schmid, G., Corain, B. Nanoparticulated Gold: Syntheses, Structures, Electronics, and Reactivities. Journal of Inorganic Chemistry. 2003, 17, 3081-3098.
- Yirka, B. Researchers Seeking To Redefine Difference Between Solids And Liquids. Phys.org, Apr 8, 2013.
- Fardin, M. Answering the question that won me the Ig Nobel prize: Are cats liquid? The Conversation, Nov 8, 2017.
- Takagi, M. Electron Diffraction Study of Liquid-Solid transition of Thin Metal Films. Journal of the Physical Society of Japan. 1954, 9, 359-363. DOI: 10.1143/JPSJ.9.359.
- Sun, J., et al. Liquid-like Pseudoelasticity Of Sub-10-nm Crystalline Silver Particles. Nature Materials. 2014, 13, 1007-1012.

It should be noted though, that below 2 nm, gold nanoparticles enter a non-scalable, cluster regime. The melting point function breaks down and the packing structure of the Au atoms is usually different from fcc. This gives entirely different melting behavior.
Thanks for this helpful input! One thing that I did not mention in the blogpost is that the described melting behavior (Figure 6) are for “ideal gold nanoparticles”, which are gold nanoparticles without any stabilization of surface ligands. For gold clusters you mentioned , I believe, are mostly stabilized by thiolated ligands in practice, which lowers the surface energy of the nanoparticles and gives different melting behavior.