These days we are all familiar with the concept of recycling, which for most of us involves the weekly ritual of placing waste materials such as bottles, cans, and cardboard into oversized, colored recycling bins and placing them outside the house. After pick up, these recyclables are transported to collection centers where they are sorted, cleaned, and converted into new materials destined for use in manufacturing.
One of the principal benefits of recycling is that it reduces energy consumption by reusing raw materials that would otherwise be thrown away as trash. Recycling also helps to conserve valuable natural resources and reduces the amount of waste sent to landfills and incineration facilities. This not only lowers costs, but also helps to protect the environment by reducing (for example) greenhouse gas emissions from incinerating plastics or the leaching of toxic chemicals from landfills. But can we recycle nanomaterials or turn traditional waste into nanomaterials?

Nanomaterials possess unique chemical and physical properties that make them desirable to use in many consumer products. This has led to a major increase in the number of household products that incorporate nanomaterials. Examples include gold nanoparticle catalysts and inorganic quantum dots found in TV and computer displays. An inevitable consequence of this surge in use is that nanomaterials are increasingly prevalent in waste streams – the more we buy something, the more it gets thrown out!
To date, only a few recycling and re-use strategies have been developed for nanoparticles.1 Designing these strategies is challenging because, in order for them to be practical, they have to be relatively simple, cheap, fast, and energy efficient. The most common recovery method used so far involves using magnets to separate iron-containing nanoparticles from complex mixtures, including wastewater.2, 3 Several methods have also been developed for the extraction, separation, and re-use of expensive gold nanoparticles from different liquids.4
A new variant on the concept of nanoparticle recycling which has been recently garnering interest is the idea of repurposing traditional waste materials into nanomaterials. For example, plastic bags have been used to create carbon dots, which are small carbon nanoparticles (less than 10 nm in size) with interesting optical properties and potential applications as imaging agents. These carbon dots were made by simply cutting the bags into small pieces and heating them in a solution of hydrogen peroxide, a household bleach you can find in your first aid cabinet.5 (Colleagues from the CSN have recently published an article in the Journal of Chemical Education about how high school classrooms can make carbon dots.6)

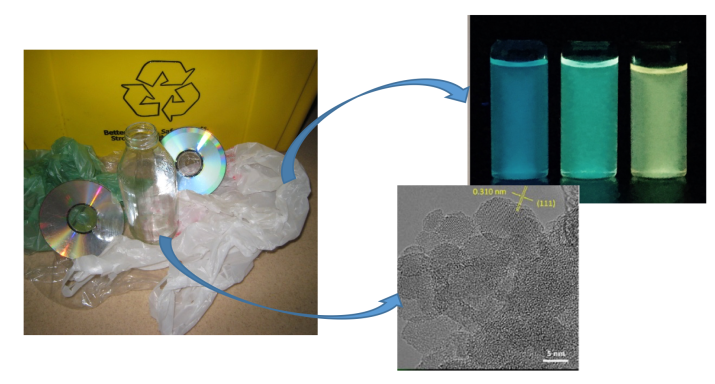
Another waste repurposing method is to heat discarded compact discs (CDs) in a furnace along with sand to create silicon carbide nanoparticles, a nanomaterial with outstanding thermal, chemical, and mechanical properties. The use of CDs is noteworthy because, with increasing urbanization in the world, electronic waste like CDs is estimated to be accumulating at three times the rate of normal household trash.7
In addition to electronic waste, glass is another common household product that accumulates in our landfills by the tons (even though it is recyclable). Like sand, glass is made of a mixture of silicon and oxygen known as silica. A research group at UC Riverside has recently devised a way to convert this waste glass (silica) into silicon nanomaterials that can be used to store energy in batteries.8 The method is relatively straightforward; glass bottles are first crushed into small pieces, cleaned with isopropanol (a common ingredient in antiseptics, disinfectants, and detergents), and mixed with salt. This mixture is then heated in a furnace in the presence of magnesium, producing silicon nanoparticles. The magnesium enables the silica to be converted into silicon in the furnace, while the salt helps to adsorb the heat produced in this chemical reaction and prevents the silicon nanoparticles from combining into a much larger (and less useful) piece of silicon. The silicon nanoparticles formed in this process can be used as an active and effective energy storage component in a lithium ion fuel cell battery, an increasingly popular lightweight and rechargeable type of battery that is replacing the more traditional, bulky, lead-acid batteries found in most cars. (See our previous posts about lithium ion batteries and electric car batteries.)
The intrinsic value of nanoparticles and the need to recover and re-use these high value materials has provided the motivation for nanoparticle recycling and the repurposing of traditional waste into nanoparticles. If successful, these two green chemistry initiatives will certainly help to reduce energy consumption and minimize waste accumulation if they can be implemented on a sufficiently large scale. For these reasons, nanoparticle recycling in its various forms is gaining traction as a topic of conversation in the scientific community, one whose importance seems certain to increase in the coming years.
REFERENCES
- Deep, A.; Kumar, K.; Kumar, P.; Kumar, P.; Sharma, A. L.; Gupta, B.; Bharadwaj, L. M., Recovery of Pure ZnO Nanoparticles from Spent Zn-MnO2 Alkaline Batteries. Environmental Science & Technology 2011, 45 (24), 10551-10556. DOI: 10.1021/es201744t.
- Myakonkaya, O.; Guibert, C.; Eastoe, J.; Grillo, I., Recovery of Nanoparticles Made Easy. Langmuir 2010, 26 (6), 3794-3797. DOI: 10.1021/la100111b.
- Myakonkaya, O.; Hu, Z. Y.; Nazar, M. F.; Eastoe, J., Recycling Functional Colloids and Nanoparticles. Chemistry-a European Journal 2010, 16 (39), 11784-11790.
- Pati, P.; McGinnis, S.; Vikesland, P. J., Waste not want not: life cycle implications of gold recovery and recycling from nanowaste. Environmental Science-Nano 2016, 3 (5), 1133-1143. DOI: 10.1039/C6EN00181E.
- Hu, Y. P.; Yang, J.; Tian, J. W.; Jia, L.; Yu, J. S., Green and size-controllable synthesis of photoluminescent carbon nanoparticles from waste plastic bags. Resource Advances 2014, 4 (88), 47169-47176. DOI: 10.1039/C4RA08306G.
- Pham, S.; Kuether, J.; Gallagher, M.; Tapia Hernandez, R.; Williams, D.; Zhi, B.; Mensch, A.; Hamers, R.; Rosenzweig, Z.; Fairbrother, H.; Krause, M.; Feng, Z.; Haynes, C. Carbon Dots: A Modular Activity To Teach Fluorescence and Nanotechnology at Multiple Levels. Journal of Chemical Education. 2017, 94 (8), 1143-1149. DOI: 10.1021/acs.jchemed.6b00995.
- Rajarao, R.; Ferreira, R.; Sadi, S. H. F.; Khanna, R.; Sahajwalla, V., Synthesis of silicon carbide nanoparticles by using electronic waste as a carbon source. Materials Letters 2014, 120, 65-68. DOI: 10.1016/j.matlet.2014.01.018.
- Li, C. L.; Liu, C.; Wang, W.; Mutlu, Z.; Bell, J.; Ahmed, K.; Ye, R.; Ozkan, M.; Ozkan, C. S., Silicon Derived from Glass Bottles as Anode Materials for Lithium Ion Full Cell Batteries. Scientific Reports 2017. DOI: 10.1038/s41598-017-01086-8.
