The Swiss Renaissance physician, alchemist and founder of toxicology Philippus Aureolus Theophrastus Bombastus (!) von Hohenheim, better known as Paracelsus (Figure 1), said it well: “All substances are poisons; there is none that is not a poison. The right dose differentiates a poison and a remedy.” Or more compactly, “the dose makes the poison.”

Researchers at the Center for Sustainable Nanotechnology are often asked whether this or that nanomaterial is toxic. This is an important question and one we’re quite interested in (you can read a couple of our earlier posts on the subject here and here). The toxicity of a compound is a measure of how hazardous it is to living things. Based on Paracelsus’ adage we would have to consider all substances toxic at some level, but that’s not usually what people mean when they ask about nanoparticle toxicity.
Let’s put things in perspective. When toxicologists conduct toxicity studies one of the first kind of experiments they do is expose the test organisms (such as water fleas, zebrafish, or rats) to the substance at a range of concentrations to see when they observe effects. Those effects might include death (pretty severe!), inhibition of growth, increase in tumor production, or increase in malformations as the organisms grow. The results are plotted as effect vs. concentration (or dose). Such a plot is referred to as a dose-response curve (Figure 2).
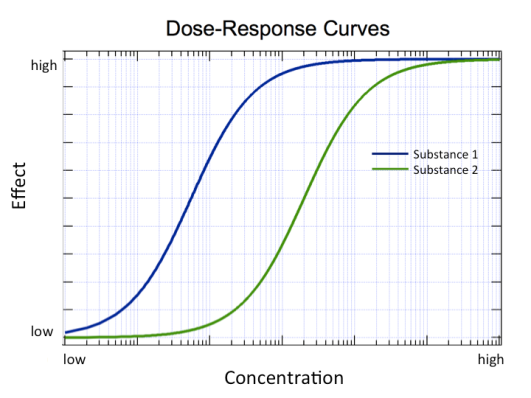
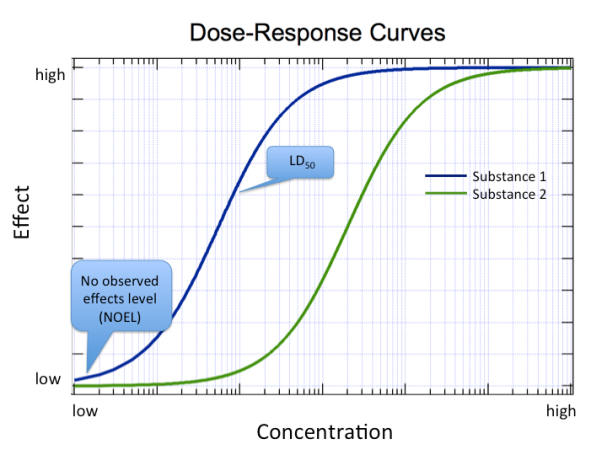
One commonly reported metric is the concentration (or dose) at which half the population of test organisms exhibits the effect of concern. For example, the median lethal dose (LD50) is the dose that kills half the population of test organisms. As you might expect, the lethal dose depends on the route of exposure (for example, was the exposure by ingesting, inhaling, or touching the skin?). Gasoline is much less hazardous spilled on your skin than if you were to drink it! The lethal dose also depends on whether you are exposed to the chemical all at once (acute exposure) or regularly over a long period of time (chronic exposure). The lethal dose also depends on the organism. For example, humans are more resistant to the effects of dioxin, a type of environmental pollutant, than are guinea pigs.1
With that background, let’s take a look at the toxicity of some common and not-so-common chemicals. Figure 4 shows me holding an acute oral lethal dose of table salt (NaCl) in a graduated cylinder in my right hand. That’s 2.76 cups, approximately how much it would take to kill 50% of people if ingested all at once (an unpleasant prospect). By way of contrast, in my left hand I am holding a lethal dose of sodium arsenite (Na3AsO3). The lethal dose of Na3AsO3 is only 2.87 g. In Table 1 below, I’ve summarized acute oral lethal doses for humans for a number of compounds. The LD50 values range from 2.1 kg (4.6 lbs) of sugar to the most acutely lethal toxin known, botulinum toxin produced by the bacterium Clostridium botulinum and now used to prevent the development of facial wrinkles (BoTox). The acute oral lethal dose is only 7 nanograms (or 0.000000000007 kg)!

| Table 1. Acute Oral Lethal Doses for Humans2 | |||
|
Substance |
LD50 (mg/kg)a | Lethal Oral Exposure (g)b |
Comments |
| sucrose | 30,000 | 2,100 (10.5 cups of granulated sugar) | Sugar, refined from sugar cane and sugar beets |
| ethanol | 7,000 | 490 (10.3 L beer, 5.2 L wine, 1.6 L vodka)c | Alcohol in beer, wine, etc. |
| sodium chloride | 3,000 | 210 (2.76 cups Morton table salt) | Table salt |
| caffeine | 200 | 14 (78× 8 oz. cups of brewed coffee)d | Occurs naturally in cocoa, coffee |
| nicotine | 50 | 3.5 (14.6 packs of cigarettes)e | Occurs naturally in tobacco |
| vitamin D | 10 | 0.7 (70× 400 IU capsules) | Essential part of human diet |
| cyanide | 10 | 0.7 (27.8 kg almonds) | Naturally occurring in almonds, apple seeds; industrial chemical |
| alfatoxin | 0.003 | 0.00021 (210 µg) | Formed by fungi on grains and nuts |
| botulin | 0.00001 | 0.0000007 (700 ng) | Formed by bacteria in improperly canned food |
| a These LD50 values are based on oral ingestion by rats and represent the single dose that kills 50% of the treated animals within 14 days of exposure. The LD50 values are expressed as mg substance per kg body mass. b Assumes 70 kg body mass (~154 lb. body weight) c Assumes ethanol contents of 6% for beer, 12% for wine, and 40% for vodka) d Assumes 180 mg per 8 oz. cup (range is 95-200) e Average nicotine content of 12 mg per cigarette; 20 cigarettes per pack |
|||
When determining whether a nanomaterial poses a risk, we first have to understand the difference between risk and hazard. In other words, we need to consider both its toxicity (hazard) AND one’s exposure to it. As an example, think about the bleach you may have at home (Figure 5). When it is sitting on the shelf it is hazardous, but does not pose a risk. If you were exposed to it (whether by ingesting, inhaling, or having it touch your skin), then there could be a risk.

So if exposure does not occur, there is no risk. In the case of nanomaterials, quantum dots used for backlighting in television displays may contain the toxic metal cadmium, but if you don’t come in contact with this material, you are not at risk of the toxic effects of the cadmium. Most people won’t be exposed to quantum dots directly from their televisions, but what about nanoparticles in the environment in general? Unfortunately, at present we do not know for certain the levels of engineered nanomaterials in the environment. Scientists have made some rough estimates based on the amounts of nanomaterials that are currently used in various industries, estimates for future production and use, and predictions about how (and how much of) those materials might find their way into the environment.3 The good news is that in most cases, the concentrations of nanomaterials that have been shown to cause adverse effects on organisms is generally higher than the amounts estimated to be in the environment right now. The less good news is that concentrations of nanomaterials in the environment are predicted to continue increasing for the foreseeable future.
So, to come back to our original question – Are nanomaterials toxic? Yes, at some level they are. But do they pose a risk? That depends on the exposure. So far exposure via the environment seems small as far as we know. One goal of the Center for Sustainable Nanotechnology is to improve our understanding of how nanoparticles may get into the environment and what happens after that exposure occurs.
* With apologies to Friedrich Nietzsche (and Richard Strauss)
EDUCATIONAL RESOURCES
- NOVA: “Cancer Warrior” lab activity (Grades 5-12)
- NISEnet/National Nanotechnology Infrastructure Network: The Effects of Gold and Silver Nanoparticles on Brine Shrimp: A Toxicology Study (high school)
REFERENCES
- Korkalainen, M. et al. The AH Receptor of the Most Dioxin-Sensitive Species, Guinea Pig, Is Highly Homologous to the Human AH Receptor. Biochemical and Biophysical Research Communications, 2001, 285, 1121-1129. DOI: 10.1006/bbrc.2001.5317
- Adapted from Trautmann. Assessing Toxic Risk, 2001 & Chaouali et al. Potential Toxic Levels of Cyanide in Almonds (Prunus amygdalus), Apricot Kernels (Prunus armeniaca), and Almond Syrup. ISRN Toxicology, 2013. DOI: 10.1155/2013/610648
- Sun, T. Y. et al. Dynamic Probabilistic Modeling of Environmental Emissions of Engineered Nanomaterials. Environmental Science & Technology 2016, 50, 4701-4711. DOI: 10.1021/acs.est.5b05828

[…] original en inglés por Joel Pedersen Traducido por Jeremy […]