According to the US National Fire Protection Association, nearly 20 percent of home fire deaths between 2006 and 2010 occurred in fires where upholstered furniture was the first item to ignite.1 In order to try to prevent this, many materials incorporate a flame retardant: a mixture of chemicals that is added to materials such as plastics and textiles in order to inhibit, suppress, or delay the material’s destruction when it is exposed to fire.2 Flame retardants are found in many everyday materials, from textiles in airplane seat cushions and military uniforms to the urethane foams in infant mattresses to plasticizers in construction and automotive materials.

The Current State of Affairs – Polybrominated Diphenyl Ethers
Some of the most common flame retardants use a group of chemicals called polybrominated diphenyl ethers (PBDEs). However, PBDEs have been found to be recalcitrant once they enter the environment – in other words, they persist and accumulate rather than dispersing. PBDEs have been detected in blood and breast milk throughout the U.S. population, as well as in wildlife.3 Prenatal exposure to PBDEs alters fetal brain development and has been linked to delayed physical development,4 reduced IQ, and attention deficit.5 These flame retardants can also exhibit endocrine disruption6 and have been implicated in contributing to infertility.7 As a result of the human health and ecological impacts, the production and use of PBDEs are currently being phased out in the United States.
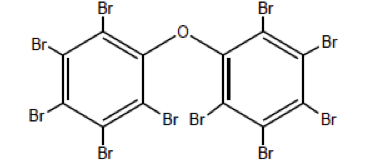
What does all of this have to do with nanotechnology? Well, there are a range of alternative flame-retardant technologies, including nanomaterials, that might work as replacements for PBDEs, but they need to be evaluated before we start using them in commercial products. As part of this effort, the Environmental Protection Agency (EPA) recently commissioned a study to assess the feasibility of using carbon nanotubes (CNTs) as a potential replacement for PBDEs as flame retardants in upholstery textiles.8
What are Carbon Nanotubes?
Carbon nanotubes (CNTs) are tubular cylinders of carbon atoms that are usually a few microns long and a few nanometers wide. CNTs consist of a hexagonal array of carbon atoms rolled up into a long, thin, hollow cylinder (see figure below); this arrangement means that they have extremely high surface areas and are many times longer than they are wide (typically 100-1000 times).9
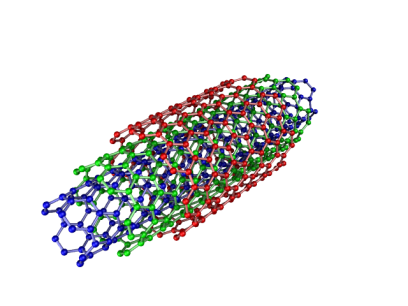
Two structural forms of CNTs exist: single-walled (SWCNTs) and multi-walled (MWCNTs) nanotubes. A SWCNT is a single, rolled up graphene sheet normally capped at the ends, while a MWCNT includes two or more concentric cylinders of carbon held together by van der Waals forces.10 CNTs’ unique structures give rise to a combination of physical and chemical properties, including remarkably high mechanical strength (e.g. tensile strengths 100 times larger than steel), combined with electrical and thermal conductivities similar to that of diamond and copper, respectively.11,12 In the past, CNTs were so expensive that it was impractical to use them (~ $1000 per gram for SWCNT), but MWCNTs can now be mass produced for only $100 per kilogram. This means that MWCNTs are becoming the most viable, cost effective choice for some large scale commercial applications. Indeed, cost is increasingly less of an obstacle for the commercialization of most nano-enabled products.
How Do Flame Retardants Work?
While each flame retardant is unique, they pretty much all act by one or more of three general mechanisms: A) They swell or promote char when they burn, forming a barrier layer to protect the underlying material or to smother the flame.2 B) They generate by-products (solid or gas) that slow down the heat released during combustion, effectively slowing a material’s burn rate and the rate the fire spreads. C) They release short-lived molecules called free radicals that react with the material and other combustion by-products in order to slow down or stop the flame. Some flame retardants are designed to take advantage of two or all three of these mechanisms at once.

Research has shown that when MWCNTs are added as coatings to upholstery textiles, they loosely bind together to form a barrier layer. Scientists at the National Institute of Standards and Technology (NIST) have shown that CNTs can be used to create a coating which reduces the flammability of foam commonly used on upholstery by 35 percent.14 CNTs also provide their flame retardant effect in smaller concentrations compared to other fillers. This means that fewer CNTs are required to achieve the same effect and they are therefore less visible in the final product. Another advantage of CNTs is that they can increase the flame retardant properties of a material while at the same time enhancing its material strength or providing an electrostatic discharge effect (i.e. they’ll shock you less in the dry winter weather). The only major technological hurdle left is to figure out how to effectively disperse the CNTs throughout the materials for maximum benefit. But that may not be much of a barrier: the CNT manufacturing company Nanocyl is already marketing MWCNTs for use as flame retardants in textiles such as couch coverings and curtains.
The Current Roadblock
One downside to all of this is that using MWCNTs as flame retardants in upholstery textiles could result in more MWCNTs getting released into the environment. There is a lack of consensus about the health and safety effects of CNTs, which has fueled concern over the fear that we may be jumping “out of the frying pan into the fire” by replacing one toxin with another that may turn out to be even more hazardous.3 These concerns seem likely to stall any larger scale commercialization of this technology for the moment. What is needed to fill this information vacuum and move the discussion forward is detailed scientific information comparing of the pros and cons of replacing PBDEs with MWCNTs. This includes information on the exposure routes and release rates of these two fire retardants during their life cycles, as well as detailed toxicity data and manufacturing cost comparisons. Unfortunately, until a reliable side-by-side comparison based on quantitative metrics is available, this potentially positive application of nanotechnology will be stymied by uncertainty and the trepidation for manufacturers and consumers alike.
EDUCATIONAL RESOURCES
- NOVA: On Fire virtual lab
- Journal of Chemical Education classroom activity: Burning to Learn15 (may need subscription)
REFERENCES (some require subscription for full article)
- Ahrens, M. Home Fires That Began With Upholstered Furniture [report]. National Fire Protection Association. 2011. Retrieved from http://www.nfpa.org/research/reports-and-statistics/fire-causes/household-products/upholstered-furniture.
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.-M.; Dubois, P., New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Materials Science and Engineering R 2009, 63, 100-125. doi: 10.1016/j.mser.2008.09.002
- Sass, J. (Feb 24, 2014). Carbon nanotube flame retardants – two wrongs don’t make a right. Switchboard: National Resources Defense Council Staff Blog. Retrieved from http://switchboard.nrdc.org/blogs/jsass/carbon_nanotube_flame_retardan.html.
- Herbstman, J.; Sjödin, A.; Kurzon, M.; Lederman, S.; Jones, R.; Rauh, V.; Needham, L.; Tang, D.; Niedzwiecki, M.; Wang, R.; Perera, F., Prenatal Exposure to PBDEs and Neurodevelopment. Environmental Health Perspectives 2010, 118, 712-719. doi: 10.1097/01.ede.0000340573.89899.1b
- Eskenazi, B.; Chevrier, J.; Rauch, S. A.; Kogut, K.; Harley, K. G.; Johnson, C.; Trujillo, C.; Sjödin, A.; Bradman, A., In Utero and Childhood Polybrominated Diphenyl Ether (PBDE) Exposures and Neurodevelopment in the CHAMACOS Study. Environmental Health Perspectives 2013, 121, 257-262. doi: 10.1289/ehp.1205597
- Chen, A.; Chung, E.; DeFranco, E. A.; Pinney, S. M.; Dietrich, K. N., Serum PBDEs and age at menarche in adolescent girls: Analysis of the National Health and Nutrition Examination Survey 2003–2004. Environmental Research 2011, 111, 831-837. doi: 10.1016/j.envres.2011.05.016
- Harley, K.; Marks, A.; Chevrier, J.; Bradman, A.; Sjödin, A.; Eskenazi, B., PBDE Concentrations in Women’s Serum and Fecundability. Environ Health Perspectives 2010, 118, 699-704. doi: 10.1289/ehp.0901450
- Agency, U. S. E. P. An Alternatives Assessment for the Flame Retardant Decabromodiphenyl Ether (DecaBDE); United States Environmental Protection Agency: 2014; pp 1-34. Available at http://www.epa.gov/sites/production/files/2014-05/documents/decabde_final.pdf
- Chen, Q.; Saltiel, C.; Manickavasagam, S.; Schadler, L. S.; Siegel, R. W.; Yang, H., Aggregation behavior of single-walled carbon namotubes in dilute aqueous suspension. J. Colloid Interface Sci. 2004, 280, 91-97. doi: 10.1016/j.jcis.2004.07.028
- He, X.; Kitipornchai, S.; C.M., W.; Leiw, K. M., Modeling of van der Waals force for infinitesimal deformation of multi-walled carbon nanotubes treated as cylindrical shells. International Journal of Solids and Structures 2005, 42, 6032-6047. doi: 10.1016/j.ijsolstr.2005.03.045
- Robertson, D. H.; Brenner, D. W.; Mintmire, J. W., Energetics of nanoscale graphitic tubules. Physical Review B 1992, 45, 12592-12595. doi: 10.1103/PhysRevB.45.12592
- Gibson, J. M.; Ebbensen, T. W.; Treacy, M. M. J., Exceptionally high Young’s modulus observed for individual carbon nanotubes. Nature 1996, 381, 678-680. doi: 10.1038/381678a0
- Kashiwagi, T., Du, F., Winey, K. I., Groth, K. M., Shields, J. R., Bellayer, S. P., … & Douglas, J. F., Flammability properties of polymer nanocomposites with single-walled carbon nanotubes: effects of nanotube dispersion and concentration. Polymer 2005, 46(2), 471-481. doi: 10.1016/j.polymer.2004.10.087
- Kim, Y. S.; Davis, R., Multi-walled carbon nanotube layer-by-layer coatings with a trilayer structure to reduce foam flammability. Thin Solid Films 2014, 550 184-189. doi: 10.1016/j.tsf.2013.10.167
- Gettys, N.; Jacobsen, E., Burning to Learn: An Introduction to Flame Retardants. Journal of Chemical Education 2001, 78, 328A-B. doi: 10.1021/ed078p328
Correction: This post originally referred to PBDEs as polybrominated diethyl ethers rather than polybrominated diphenyl ethers. We updated the post to correct the error on 1/16/16.

I’d also love to see more about the properties of the CNT residue after burning. Kashiwagi et al describe it as being strong enough to handle without breaking. But would that be true in other cases, and would that also be true during fire fighting? The thing I’m concerned about is aerosolizing the nannotubes during those processes. I realize that may be minor compared to all the other respiratory issues during a fire (or the issues of PDBEs), but definitely needs to go into the pro/con analysis.
Howard says, “It’s a really good point and definitely something that needs more research, the idea that the char layer may be a means to release CNTs into the atmosphere once it’s formed – however, I don’t have any direct knowledge about this aspect of the flame retardant process.”