One of the largest oil spills in recent history happened in April 2010 in the Gulf of Mexico when the Deepwater Horizon oil rig exploded and sank, resulting in the blowout of the Macondo well located approximately 66 km off the Louisiana coast. Oil spills, or the accidental release of liquid petroleum into the environment, pose a major concern to the health of natural ecosystems. Marine oil spills are known to injure and kill sea birds, heavily furred mammals, and marine organisms including shellfish, corals, and dolphins. There are various sources of oil spills including tank vessels, pipelines, and directly from facilities.

According to the statistics provided by the International Tanker Owners Pollution Federation (ITOPF), which has a database for oil spills from tankers, combined carriers, and barges, over 9000 accidental spills have occurred since 1974. As per the US Environmental Protection Agency, the most common methods for containing and combating oil spills are mechanical containment (recovery methods using equipment like barriers, booms, skimmers etc.), and chemical and biological agents like dispersants (which help the oil to disperse more easily throughout the water around it) and gelling agents (which combine with oil and form a rubber-like solid for easier cleanup). With recent developments in the field of nanotechnology, nanomachines have emerged as an efficient, selective and environment-friendly option for the potential treatment of oil spills.
The Deepwater Horizon incident led to the discharge of 210 million US gallons of crude oil into the Gulf of Mexico.1

Millions of gallons of chemical oil dispersants called Corexit 9527A ® and Corexit 9500A ® were used to treat the spill.2 Dispersant molecules reduce the interfacial tension between oil and water and can break the oil slick. This helps in the transfer of the oil from the surface of water into the water column, which makes it less likely for the oil to reach the shoreline. However, recent scientific studies have raised concerns about the use of dispersants like Corexit. A study from the Georgia Institute of Technology and Universidad Autonoma de Aguascalientes (UAA), Mexico have shown that a mixture of Corexit 9500A® and oil is 52 times more toxic than oil alone to microscopic marine rotifer Brachionus plicatilis.2 Similar studies have shown that the total petroleum hydrocarbon (TPH) uptake for ghost shrimp Palaemon serenus, increases when exposed to dispersed oils obtained with Corexit, than when exposed to crude oil.3
These problems bring us to the recent development of nanomachines (like the ones discussed in this recent post) as an improved alternative to chemical dispersants for oil spill remediation. Nanomachines are nanodevices that can convert energy into movement in order to perform various tasks. They can use a range of energy sources, including bubble formation, light, and magnetic or ultrasound fields. Recently one group of researchers has developed a nanomachine that can help capture, transport and remove oil droplets.4 They used microsubmarines propelled by oxygen bubbles that are generated from the catalytic oxidation of hydrogen peroxide fuel at an inner platinum layer. The microsubmarines are coated in a layer of hydrophobic (water hating) molecules, which interact with the oil droplets, making the micromotors well equipped to capture oil droplets and transport them.

Another example of how nanotechnology can improve oil remediation is the use of micromotors based on magnesium Janus particles, which are magnesium particles coated by nanometric layers of titanium, nickel, and gold and further functionalized with a hydrophobic layer of octadecanethiol, to scavenge oil droplets in water.5 Unlike the previous example, these micromotors use hydrogen bubbles for propulsion, which come from a reaction involving reduction of water at the magnesium surface.
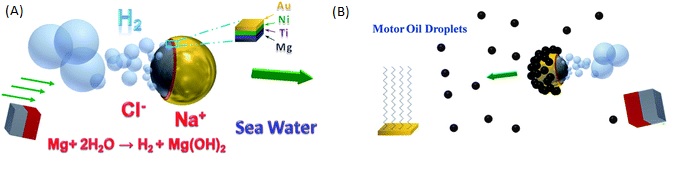
One potential issue with this motor is that the magnesium surface quickly becomes covered, or passivated, by an oxide layer and can no longer produce the reaction with water that makes the bubbles. The gold layer on the motor helps with this problem because it interacts with the chloride ions in seawater to help in removing the oxide passivation layer, through a combination of macrogalvanic corrosion and pitting corrosion processes. Like the first example, these micromotors attach to oil droplets and help in their capture and transport in water. Because these micromotors can propel in seawater all by themselves, they have the potential to be valuable in removing oil droplets from seawater.
These nano/micromotors may provide an environmentally friendly and efficient alternative for treating oil spills. In the case of the magnesium Janus micromotors, the composition of the motor and the use of seawater as the sole fuel source make them extremely biocompatible and eco-friendly. Unlike dispersants, which break up the oil slick and transfer the oil down the water column, the nano/micromotors have the potential to capture, transfer and remove oil droplets from water. Apart from the corrosion issues, these nano/micromachines also have the tendency to slow down after collecting the oil droplets. Research is ongoing to fix the problems of longevity and speed of these nano/micromotors. Unfortunately, there are no known instances of these nano/micromotors actually being used for oil spill control yet, and it isn’t clear exactly what happens to the nomachines after they attach to the oil.
The use of nano/micromotors for oil spill remediation is in its nascent stage. But it is evident from the couple of examples that we have in hand that they could have profound applications in water treatment and oil spill control and could potentially revolutionize the field of environmental remediation.
REFERENCES (subscription required for most)
1. Zukunft, P. Summary Report for SubSea and SubSurface Oil and Dispersant Detection: Sampling and Monitoring. DIANE Publishing, 2010.
2. Rico-Martínez, Roberto, Terry W. Snell, and Tonya L. Shearer. Synergistic toxicity of Macondo crude oil and dispersant Corexit ® to the Brachionus plicatilis species complex (Rotifera). Environmental Pollution 173 (2013): 5-10.
3. Gulec, I. & Holdway, D. Toxicity of crude oil and dispersed crude oil to ghost shrimp Palaemon serenus and larvae of Australian bass Macquaria novemaculeata. Environmental Toxicology 15.2 (2000): 91-98.
4. Guix, M., Orozco, J., García, M., Gao, W., Sattayasamitsathit, S., Merkoçi, A., Escarpa, A. & Wang, J. Superhydrophobic alkanethiol-coated microsubmarines for effective removal of oil. ACS Nano 6.5 (2012): 4445-4451.
5. Gao, W., Feng, X., Pei, A., Gu, Y., Li, J. & Wang, J. Seawater-driven magnesium based Janus micromotors for environmental remediation. Nanoscale 5.11 (2013): 4696-4700.

[…] Para leer el blog original en ingles, presione aquí. […]
[…] the Past,” was selected for the week of Sept 28-Oct 4; Sunipa Pramanik’s post, “Using Nano/Micromachines to Help Clean Up Oil Spills,” was selected for the week of Nov 16-22, 2014; and Alicia McGeachy’s post, […]