In Marco’s previous post, you can read how making nanoparticles is like baking – different proportions of ingredients and different processing conditions (time and temperature) can turn your batter into a pancake, bread, a biscuit, etc. But how do you come up with recipes to make nanoparticles in the first place?
In general, as I see it, there are two main ways to make nanoparticles:
1. Take a large hunk of material and drill/blast/mechanically process it into tiny nano-sized pieces. This is the top-down approach. Another way to think about this is “making big stuff smaller.”
2. Take a molecule, or a simple salt , that has the right atoms, and perform a chemical reaction to build the nanoparticle atom-by-atom. This is the bottom-up approach. Another way to think about this is “making small stuff bigger.”
No matter which way you do it, though, the trick is to control the nanoparticle size and shape!
Let’s look at examples of these approaches.
Top-Down Approach
The nanodiamond we use in our Center is made via the top-down approach. Nanodiamond is nearly pure carbon. Nanodiamond is made by detonation (explosion) or ultrasonication (using sound waves to break up materials) of bulk graphite flakes:
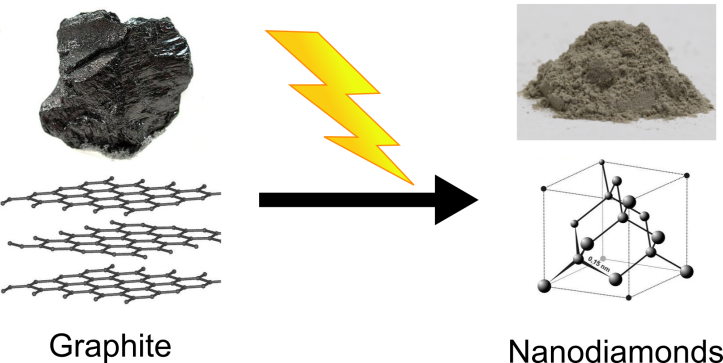
This method of synthesis converts millimeter-size flakes of graphite into nanoscale diamond particles. The process costs some energy and does not sound very controllable, but if all goes well, you will get nanodiamond particles that are about 5 nanometers in diameter. Bob Hamers’ group is working on ways to chemically modify nanodiamond particles so they will be stable in water for our biological experiments.
Bottom-Up Approach
The gold nanoparticles we have used in the Center so far are made by a bottom-up approach. We take a water-soluble form of gold, known as a gold salt, HAuCl4. This gold salt produces H+ and AuCl4– ions when dissolved in water. So you can picture these ions floating around in water, with at most one gold atom per ion. Then we add a chemical compound called a reducing agent, which converts the gold ions into elemental gold atoms. These gold atoms are not as soluble in water, and so clump together into nano-sized particles. I have illustrated this below, and I am just showing 4 gold compounds to illustrate the chemistry:

The problem here is that as those gold atoms form nano-sized lumps of gold, they have a tendency to continue to grow into bigger, non-nano-sized lumps. If we are not careful, we just form big flakes of gold:

So we need to stop the uncontrolled formation of big lumps of gold to make well-defined nanoparticles, ideally with atomic-level control, like this:
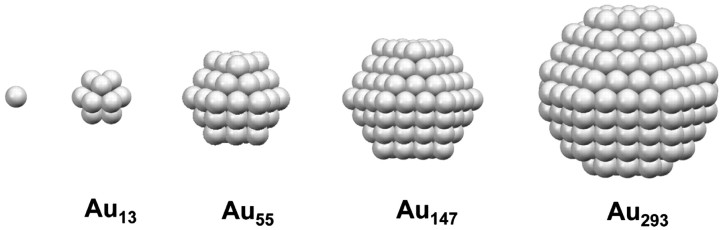
One way to do this is to put molecules that form a protective coating on the surfaces of the growing particles. These “stabilizer” molecules prevent additional gold atoms from increasing the particle size and they also prevent particles from sticking together. So, you want one end of the molecule to bind rather tightly to gold, and the other end to be water-soluble. A good example is mercaptopropylamine, which we use in the Center:

The sulfur (S) end binds to gold, losing the H on it in the process, and the NH2 group is soluble in water. For clarity, I just show one of these molecules binding to one gold atom at the surface of a tiny cluster:

If we carry out these reactions just right, we can make nice 4 nanometer diameter gold nanoparticles that are stable in water. We can make these particles in water by the gallon in our flow reactor!

We do not have a precise count of the number of gold atoms in each particle, but that is ok! We can estimate, knowing how gold atoms pack into crystals, that there are about 2000 gold atoms in one 4 nm diameter gold nanoparticle. Wondering how we do that calculation? Check this blog out later!
For now, just know there are two main ways to make particles that are nano-sized: the top-down approach as in nanodiamond formation from larger graphite chunks and the bottom-up approach as in gold nanoparticle formation from gold ions.
Related Activities:
Making Gold, Silver, and Other Nanoparticles
Exploring Materials: Nano Gold
Nanoparticle Stained Glass

Great Post!!