Everything is made of atoms. Usually these atoms are strongly connected to one another, in an amazing variety of configurations. But atoms are so tiny, how can we possibly understand the structure of matter at the atomic level?
You probably have seen pictures of molecules or materials, zoomed in to the atomic level, which show spheres of different colors connected by lines.
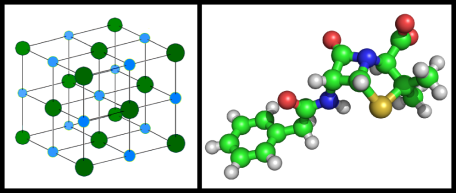
In pictures like this, the colored balls represent different kinds of atoms or ions (ions being atoms that have either lost or gained some electrons), one for each color; and the lines represent the chemical bonds that keep the atoms together.
After looking at pictures like this, you might wonder – how real is this? Do we really know the three-dimensional position of atoms in a solid substance? And, does it matter how atoms are connected to each other?
The Crystal Connection
The answer to the first question is: Yes, this is real! The classic method to determine the three-dimensional arrangement of atoms in a solid substance requires that you first obtain a pure, perfect crystal of that substance. It should be something like a cubic millimeter in size—about the size of a small grain of sand.

The perfect flat surfaces of a crystal, known as facets, actually reflect the near-perfect arrangement of the atoms that make up that crystal. These flat crystal faces tell you that the atoms, millions upon millions of them, are in fact arranged in an orderly fashion. This systematic and regular ordering is the key to allowing us to determine the position of atoms in solid substances. Now, how exactly do we do that?
Interference is the Solution
Since the 1930’s, the answer to this question has been: by diffraction.
If you send light through a sufficiently small slit, this interesting phenomenon called diffraction can occur. If the slit is small – about the size of the wavelength of light – then as the light waves pass through, they will either overlap or cancel each other (scientists would say, “constructively and destructively interfere with each other”) to yield a diffraction pattern on the other side of the slit.
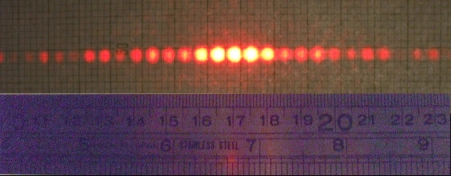
So for our crystal, we need to send in light that has wavelengths the size of atoms; the atoms in the crystal will act like a series of slits and produce a complex diffraction pattern. An average atom is about 0.1 nanometers in diameter; light with this size wavelength is in the x-ray region of the electromagnetic spectrum. If you have a nice x-ray source that provides a very specific wavelength of light, and you also have a very nice crystal, you can get very nice diffraction patterns. This technique is known as single-crystal x-ray diffraction.
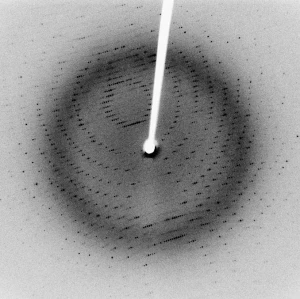
To “solve the structure,” you need to measure the positions and relative intensities of the spots in your diffraction pattern, and you can back-calculate where all the atoms were. We won’t show the math here, but it is basically algebra and trigonometry—no calculus required!
Darker spots come from heavier atoms (higher atomic weights) and lighter spots are due to lighter atoms. You cannot tell, though, what element is what. Carbon atoms and nitrogen atoms, for example, are hard to tell apart in x-ray diffraction. You would need some additional measurements to confirm which elements make up your solid substance.
Why Does Atomic Structure Matter?
Now: does it matter how atoms are arranged in a solid? The answer here too is: Yes!
The best example of this is carbon. We all know that carbon is one of the most important elements in our planet; all living things contain carbon. Pure carbon is known to us as diamond, if arranged one way in three dimensions; and graphite if arranged in another.
In diamond, all the carbon atoms have strong chemical bonds to four other carbon atoms, making perfect tetrahedra on and on throughout the crystal.
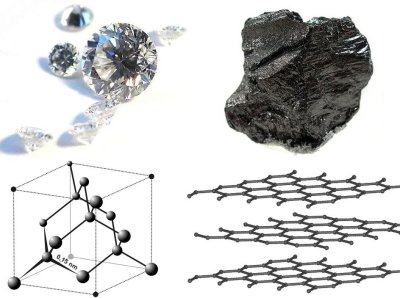
In graphite, all the carbon atoms have strong chemical bonds to three other carbon atoms, making sheets that look like chicken wire; weak forces hold the sheets together in stacks that can slide past each other easily. When you’re writing with a pencil on paper, it’s these sheets that are sliding apart to leave the graphite chunks behind as a mark on the paper.
Clearly the properties of diamond and graphite are very different. Diamond is one of the hardest materials known, is transparent to light, and does not conduct electricity at all. Graphite is soft, gray, and can conduct electricity reasonably well. Such different properties, from two substances that are composed of exactly the same kinds of atoms! And it is all due to how the carbon atoms are arranged! The ability of a solid to exist in more than one configuration like this is known as polymorphism.
The polymorphic properties of carbon don’t stop there though! There is a lot of interest in the nanotechnology community about “nanocarbons”. Nano-diamond is something new that we are working with in our Center. Will the properties of nano-diamond be just like we expect from its larger, bulk counterpart? Or, will we find surprises? You will have to come back to this blog to find out!
Pure carbon can also be found in the form of molecules that have well-defined numbers of atoms. The Nobel Prize in Chemistry in 1996 was awarded to the three chemists who discovered true molecular forms of carbon: molecules like C60 and its relatives, known as fullerenes or buckyballs. The fullerenes have properties different yet again from diamond or graphite; for example, C60 is purple and can be dissolved in oily liquids.

The fact that pure carbon has so many forms, with so many different properties, is why chemists keep doing chemistry. Even the pure elements in the periodic table are still full of surprises!
Further Exploration:
Exploratorium Diffraction Activity
Exploring the Nanoworld with LEGO Bricks: Structures at the Nanoscale
Note: This post was updated on 1/31/2020 to correct a typo in the second-to-last paragraph.

[…] nanodiamond we use in our Center is made via the top-down approach. Nanodiamond is nearly pure carbon. Nanodiamond is made by detonation (explosion) or ultrasonication (using sound waves to break up […]