Today we’ve got some important things to talk about.
I’m not talking about the how or why we’re here on this planet, or settling the great cake vs. pie debate (because clearly pie is the correct choice). No, today we’re thinking big thoughts on small things.
First things first though. Gone are the days when everything was painted in black and white. That ended when we were about 5, and we should just let that go. This world is complicated and vast. If we can agree on that much, then my question is: What the heck is going on with all the nano-hype and why are we so concerned about its sustainability?!
We’re not here to tell you that nanomaterials are always good or always bad (remember the black and white rule? We just went over it). That’s too general. That’s like saying women are bad drivers (I don’t EVEN want to hear it dudes). We want to find out the nitty gritty details of this mess, because nanomaterials are becoming more and more common in real-life where we work and play.
For example:
![[ ©2006 David Hawxhurst, Woodrow Wilson International Center for Scholars.]](https://sustainablenano.files.wordpress.com/2013/02/comsumer-products-containing-nanomaterials.png?w=450&h=243)
Like we’ve talked about in previous posts, it’s the ridiculously small size of these particles that make them act differently than what we’re used to at the human-sized scale. For example, a brick of gold you might pick up from your friendly banker is really useful for buying a new wardrobe, but is completely useless when it comes to healthcare (besides, you know, paying for it). Gold-based nanomaterials, on the other hand, actually have a ton of potential for exciting medical applications because they can be used to transport drugs to particular parts of the body or even help detect cancer.

Really, it’s both the small size and the elements that make up a nanomaterial that will dictate what it can be used for and whether it could pose any threats to our health and environment. This doesn’t mean we should be afraid of them. Nanomaterials can really improve the performance of many products. BUT, we should want to learn about them and how they interact with us and our environment so we can take advantage of all the positive benefits and prevent any hazards.
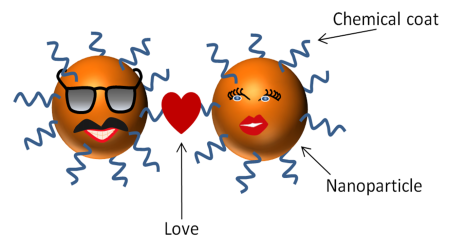
Picture the life of a nanoparticle (a type of nanomaterial that is close to spherical). It’s a pretty simple life – Dotty (the nanoparticle) is born in a lab, grows to be the proper size, then gets dressed up in fancy organic coats, and prepped and primed for her ultimate purpose. Maybe Dotty is going to work in a solar cell where she’ll spend the rest of her days basking in the sun. Maybe she’ll fall in love with her neighboring nanoparticle because he resonates with her electrons. And who doesn’t like sustainable solar energy made possible from smitten nanoparticles? They’re adorable.
But what if the unthinkable happens, and the solar panel where Dotty lives is suddenly damaged by weather/humans/confused-seagulls? Dotty and all her nanoparticle friends could leak out and be forever changed by the harsh cold world. Where will they go? And how will their properties change over time? That’s part of what we are trying to answer here.
We use lots of different methods to attack this question. Some of us prepare “tailor-made” nanoparticles to study. Some of us use lasers to see things our normal eyeballs can’t see. Some of us feed nanoparticles to bacteria or small fleas to see if they survive, or if they grow a third tail and look like something that crawled out of the Hudson River. It’s important to know what might cause materials to be toxic, so that we can design new materials to be completely harmless and still function just as well (or better!). I like you, Earth. And I like you, reader. You probably look better without a third tail…or any tail at all for that matter.
Stay tuned for all the exciting news to come!


This is incredibly well-written and explains the center, it’s purposes, and it’s goals in both a fun and simple manner. awesome job!