Did you know that scientists have used nanotechnology to develop a test for the MERS coronavirus? The results of the test can be seen by the naked eye within 10 minutes of interacting with the viral DNA, which can shorten the analysis and interpretation time.1 Current testing for the COVID-19 virus takes hours or days.2 So, if new, faster tests could be developed (and made widely available), it could facilitate much more efficient testing and tracking of infections. So how does this fast virus test work?
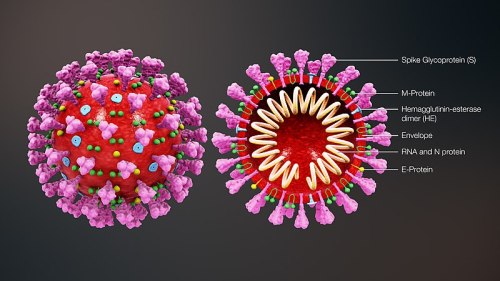
First, what are coronaviruses?
If you remember from our second episode of the Sustainable Nano podcast, Dr. MG Finn talked about how viruses are essentially entities that exist to replicate genomic information, and the vast majority of them don’t make you sick. Viruses contain DNA or RNA (two similar, but distinct kinds of genetic material that carry the identity of the virus), which they insert into host cells to hijack the cellular machinery. This means instead of doing its regular job, the cell is put to work making more virus. In some cases, like with coronaviruses, this will make your cells get sick or die.

COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is a member of Coronaviridae virus family. These viruses have characteristic spikes on their protective outer layer that look like the projections from a crown. This type of virus causes respiratory and intestinal infections in animals and humans, and they were not considered especially harmful to humans until the outbreak of Severe Acute Respiratory Syndrome (SARS-CoV) in 2002-2003 and MERS-CoV in 2012.3

How do we test for coronaviruses?
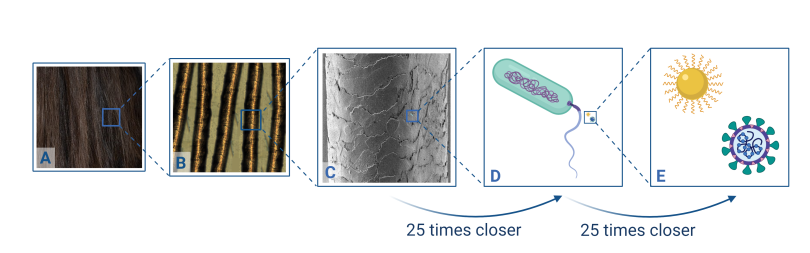
Just how small are coronaviruses? They are about 120 nm in diameter, which is way too small to see even with a regular microscope. It’s about 25 times smaller than an E. coli bacterium (~2.5 µm long), which is about 25 times smaller than the diameter of average human hair (~62.5 µm wide). Many of the gold nanoparticles we use in CSN research are about the same size as the virus, around 100 nm.
So how can we test for these viruses if we can’t see them? The method of choice for diagnosing human coronaviruses is polymerase chain reaction (PCR). This technique allows scientists to make a bunch of copies of genetic information like DNA, so in just a few hours a single copy of DNA can become a billion copies, which is enough to let us figure out what it is. This replication process happens by adding small, virus-specific segments of DNA, called primers. The primers attach to the viral DNA and allow a starting point for the copying process to occur and ensure only the viral DNA gets copied. Scientists can monitor how many copies of DNA are made in each reaction by adding a dye that fluoresces (lights up) every time a copy is made. So as more and more copies are made, the fluorescence signal gets brighter and brighter. If the virus contains RNA instead of DNA, then there’s an extra step called reverse transcription (RT) to convert the RNA to DNA before replication… which gives us the technique called RT-PCR.

RT-PCR enables us to measure the amount of coronavirus RNA that we started with by monitoring the copying process and fluorescent signal, as shown in the infographic below. If we know how much fluorescent signal we ended with and how many reactions were done, it’s then possible to figure out how many copies of viral DNA we started with!
As an analogy, imagine that your city is experiencing a power outage, and everything is dark until you decide to light some candles. Then if two neighbors see your candles and light their own, and then in turn two of each of their neighbors light candles, and so on, pretty soon the whole city will be lit up. But it will take a while! If instead of just your house starting things off, there were ten houses who started the candle trend, the city will get lighter and brighter much faster. Similarly, when using RT-PCR, if you have a lot of the viral RNA initially, the fluorescent signal will get bright quickly to help determine that you are infected. If you didn’t have any viral RNA, the fluorescent signal won’t show up at all, confirming that you are not infected. The infographic below also outlines several drawbacks of this technique, including chemical and instrumentation issues and shortages, time (it takes several hours to get results), and risks of false positive and false negative results.

How can nanotechnology help detect coronaviruses?
The best way to contain a rapidly spreading disease is early diagnosis with a test that is quick, accurate, and doesn’t require specialized training. To try to develop such a test for COVID-19, researchers are taking advantage of the optical properties and surface chemistry of gold nanoparticles to detect coronaviruses, and have already seen promising results with MERS-CoV. In a previous blog post, we explained that the optical properties of gold nanoparticles come from localized surface plasmon resonance (LSPR), giving them their unique color:
LSPRs are wavelengths of light where certain electrons in the gold resonate with the incoming light waves, so that the nanoparticle can absorb the incoming light and those electrons actually end up moving based on where the peaks and valleys of the light waves are.
Meng Wu, “What gives gold nanoparticles their color?”

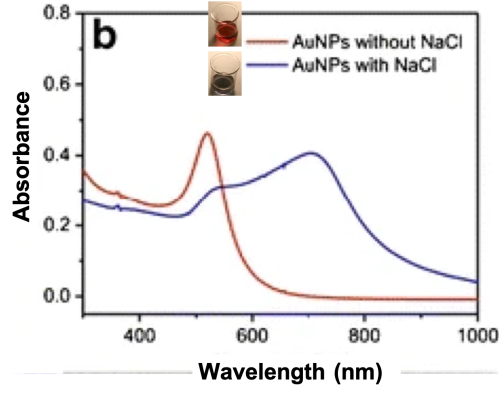
This makes gold nanoparticles have different colors depending not only on their size and shape, but also on external conditions such as positive or negative charge, acidity, and any biomolecules that are stuck to them. In the red liquid on the left below, repulsive forces are preventing gold nanoparticles from “sticking” to each other since they are of the same charge (just like how the same poles of magnets repel each other, similar charges on nanoparticles can cause them to repel each other). But once a salt is added to the solution (sodium chloride or table salt in the case shown on the right below), the repulsive forces are reduced, allowing the particles to interact and “stick together.” Once they are aggregated like this, they act like bigger particles and therefore look more blue-gray instead of red.

Why does the color change? The aggregation of the nanoparticles causes a shift in that LSPR wavelength of how the electrons absorb incoming light, and that leads to a change in the color that we see from red to grayish-blue.4
Using this principle, Kim and coworkers developed a colorimetric test for MERS-CoV.1 The researchers designed single-stranded DNA (ssDNA) probes which target regions of DNA that are unique to MERS-CoV. The probe is designed to pair complementarily to the MERS-CoV ssDNA. In addition to the ability to target the specific DNA of MERS-CoV, the probe has a chemical modification called a thiol group (-SH) added to its end.
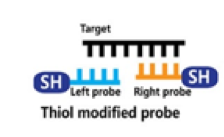
When these thiol-modified probes find their matching pair, they can react together to form disulfide (S-S) bonds and produce a self-assembled complex. On the other hand, if a sample of DNA from a patient without MERS-CoV is mixed with these probes, the probes won’t be able to find their matching partner, and can’t form any complexes.

After interacting with the DNA (and forming complexes or not depending on whether there are any matching targets), the mixture is incubated with a gold nanoparticle solution. As mentioned above, the addition of different chemicals can change the aggregation of gold nanoparticles and therefore the color we see. The reason this is important is that big DNA complexes can protect the gold nanoparticles from aggregating when a salt is added. Without the target DNA and the big complexes, the gold nanoparticles will aggregate when the salt is added, causing a significant color change.
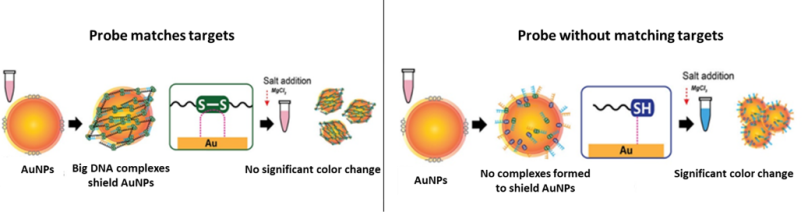
The researchers designed their probes for two target genes found in MERS-CoV, called upE and ORF1a. That means the protective, self-assembled complexes only form when the probes match with those complementary parts of the MERS-CoV DNA. But the scientists had to be sure that these probes only make the big complexes when they interact with MERS-CoV DNA and not with other viruses. So they used tobacco mosaic virus (TMV) as a negative control, meaning that they checked to make sure there was no reaction with TMV, since that virus doesn’t contain any DNA that would match with the probes.
To test this new method, the researchers incubated their probes with upE, ORF1a, and TMV DNA, and then added gold nanoparticles. Then they added the salt solution to each mixture to see if the ones with TMV changed color significantly while the MERS-CoV ones stayed relatively the same. As seen in in the graphs below, when the probe finds its complement targets, the gold nanoparticles are protected and the color (LSPR wavelength) does not shift its peak significantly (top two lines, ORF1a and upE). But when the probes can’t find their match (bottom line, TMV) the LSPR shifts to the right towards higher wavelengths of the spectrum and we can see a greater color change with our naked eyes.

To make sure these results were consistent, the scientists repeated the experiment three times, and confirmed that the color peak changes were significantly different for MERS-COV compared with the tobacco viruses.
By using the optical properties of gold nanoparticles, this new probe can confirm the presence of MERS-CoV by naked eye within 10 minutes after interacting with the viral DNA, even if there is only small number of viral molecules to start. This is just one way that nanotechnology can help combat disease like the coronavirus. There are also other studies showing the potential of other nanoparticles and nanocomposites like carbon dots, graphene oxide-silver, hydrogels in hindering viral activity!5-7 But those will have to be topics for future posts.
REFERENCES
- Kim, H. et al. Development of Label-Free Colorimetric Assay for MERS-CoV Using Gold Nanoparticles. ACS Sensors 2019, 4 (5), 1306–1312. doi: 10.1021/acssensors.9b00175.
- FDA Press Release. Coronavirus (COVID-19) Update: FDA Issues first Emergency Use Authorization for Point of Care Diagnostic. March 21, 2020.
- Cui, J., Li, F., & Shi, Z. L. Origin and Evolution of Pathogenic Coronaviruses. National Reviews in Microbiology, 2019, 17 (3), 181–192. doi: 10.1038/s41579-018-0118-9.
- Pluchery, O., Remita, H., & Schaming, D. Demonstrative Experiments about Gold Nanoparticles and Nanofilms: An Introduction to Nanoscience. Gold Bulletin. 2013, 46 (4), 319–327. doi: 10.1007/s13404-013-0122-9.
- Du, T. et al. Antiviral Activity of Graphene Oxide-Silver Nanocomposites by Preventing Viral Entry and Activation of the Antiviral Innate Immune Response. ACS Applied Bio Materials. 2018, 1 (5), 1286–1293. doi: 10.1021/acsabm.8b00154.
- Dey, P. et al. Multivalent Flexible Nanogels Exhibit Broad-Spectrum Antiviral Activity by Blocking Virus Entry. ACS Nano, 2018, 12 (7), 6429–6442. doi: 10.1021/acsnano.8b01616.
- Villeret, B. et al. Silver Nanoparticles Impair Retinoic Acid-Inducible Gene I-Mediated Mitochondrial Antiviral Immunity by Blocking the Autophagic Flux in Lung Epithelial Cells. ACS Nano, 2018, 12 (2), 1188–1202. doi: 10.1021/acsnano.7b06934.
