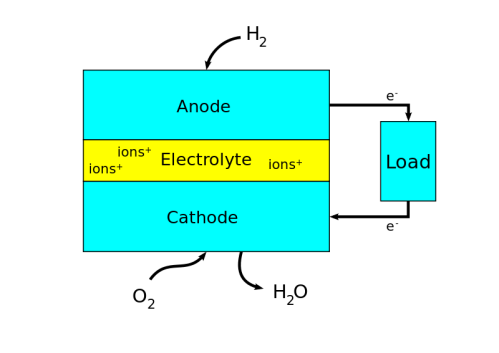
Fuel cells are a technology for transforming the chemical energy of a fuel into electricity using redox reactions (usually combining hydrogen fuel with oxygen from the air). They are different from most batteries, because they require a constant source of oxygen and fuel to sustain the chemical reaction. In batteries, the energy comes from metals and their oxides or ions that are already stored inside the battery; when those materials run out, the battery dies. As long as a fuel cell has a supply of oxygen and fuel, it can keep producing power continuously.

The concept of fuel cells is not new. The first fuel cell was invented by William Grove way back in 1839. Originally dubbed the “gas battery,” the initial design has been improved upon over the decades since to become our modern fuel cell. These innovative powerhouses have been used in various applications over the years, from NASA spacecraft to FCEVs (fuel cell electric vehicles).

What is new about modern fuel cells is that scientists have used nanotechnology to develop vastly superior fuel cells designed to drive us into the future of automotive fuel cells and beyond. With the goal of zero-emissions vehicles and elimination of our reliance on fossil fuels, nanotechnology is improving the design and durability of fuel cells and may eventually even lower the cost of production.
How Nanotechnology is Used in Fuel Cell Production
Nanotechnology is now being implemented to optimize the materials, fuels, and processes involved in fuel cell production, for advanced performance, durability, and hydrogen storage.1
Like a combustion engine, fuel cells require fuel and air to power a vehicle. For example, in hydrogen fuel cells, hydrogen flows to one side of the cell as air is pumped through a membrane to the other side. Hydrogen is then oxidized at the anode and converted into protons, which flow to the cathode, forming water. The catalyst (most often platinum) in the anode and cathode facilitates the spontaneous reaction that produces electricity with 40-60% efficiency and no dangerous waste products. This electrochemical reaction emits only water and heat.
Platinum Nanotechnology – The Same but Better
The ability to break down particles of platinum, a very expensive raw material, into nanoparticles can make catalysis much more efficient while reducing the overall amount of platinum required.
A research team at the Technical University of Munich recently reduced the size of platinum particles to the point of actually doubling catalytic performance when compared to those currently used in commercial fuel cell applications. Using computer-generated models, the team discovered that a cluster of just 40 platinum atoms is enough to result in lots of activity relative to the tiny mass of material in the catalyst.2
Nano-reactor Biofuel – Harnessing the Power of Nature
Removing costly platinum (even 40 atoms’ worth) from the hydrogen fuel cell equation, scientists at Indiana University developed a modified enzyme protected by a “capsid” protein shell3 of a bacterial virus that also catalyzes hydrogen formation.
The team claims their new material produces hydrogen gas without the need for platinum in the reaction. Using fermentation, this nano-reactor is both biodegradable and sustainable. Supported by the U.S. Department of Energy, the next step in the project will be incorporating the bio-based technology into a solar-powered energy production system.
Cobalt-graphene catalyst – Slower to Start but Faster to Finish
Another new catalyst that might be able to replace platinum in fuel cells has been developed by researchers at Brown University.4 They combined graphene, a honeycomb-like sheet of carbon just one atom thick, with cobalt to form a new nanomaterial that could offer the best reduction performance of any platinum replacement in fuel cell technology. While its properties are similar to platinum, the cost is far less.
One drawback is a slower start to the catalytic reaction for oxygen reduction in comparison to that of platinum. However, the cobalt-graphene catalyst has shown itself to degrade slower than platinum, offering more stability over time. In fact, the cobalt-graphene catalyst outperformed its platinum counterpart by 10% over a 17-hour testing period.
PEMFCs – Proton Exchange Membrane Fuel Cells
In addition to catalysts, nanotechnology has helped significantly improve fuel cell membranes, including a proton exchange membrane (PEM) developed by researchers at the University of Illinois.5 Their proton exchange membrane consists of a layer of porous silica over a 5-nanometer in diameter thick silicon layer. Water stays in the nanopores of the silica layer to combine with acid molecules.
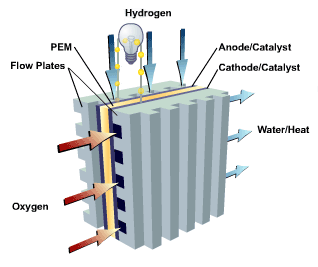
The acidic solution formed by the water in the silica nanopores helps hydrogen ions pass through the membrane. This results in a membrane that performs 100 times better in low humidity conditions than conventional fuel cell membranes.
Nanotech-Assisted Hydrogen Storage
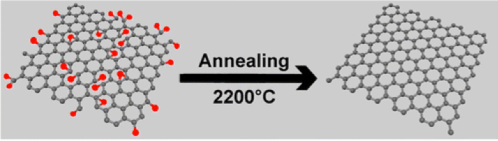
Graphene is useful for another aspect of fuel cell technology: hydrogen storage.6 Hydrogen is the most promising fuel alternative in the automotive industry due to its clean-burning characteristics. However, hydrogen fuel cells have certain limitations, such as their efficiency, size, and challenges with making sure the stored hydrogen is safe, since it is very flammable. Luckily, these challenges are slowly being overcome. Using techniques such as annealing (which involves heating a material and then cooling it slowly to strengthen it and remove impurities) researchers at Rensselaer Polytechnic Institute7 boosted the already high-bonding energy of hydrogen to the carbon in graphene sheets. The very high surface area exposure of graphene allows for greatly increased storage of hydrogen in fuel cells.
These are just a few of the most exciting ways nanotechnology is opening new frontiers in the development of hydrogen fuel cells. With each new discovery, society comes closer to a fossil fuel-free future.
Giles Kirkland is an automotive industry researcher and writer. He focuses on the technological, scientific and sustainable aspects of the automotive. As the world evolves faster than ever, he enjoys keeping track of all current developments and sharing his knowledge and experience with other motoring and technology enthusiasts across the globe. Feel free to read more articles by Giles on Twitter and at Oponeo, or contact him at [email protected].
REFERENCES
- Gondal, I. The role of nanotechnology in fuel cells and renewable energy. Technology. ISESCO JOURNAL of Science and Technology. 2015, 11(19) 72-75. Available on ResearchGate.
- Garlyyev, B. et al. Optimizing the Size of Platinum Nanoparticles for Enhanced Mass Activity in the Electrochemical Oxygen Reduction Reaction., Angewandte Chemie. 2019, 58(28), 9596-9600. doi: 10.1002/anie.20190492
- Jordan, P. et al. Self-assembling biomolecular catalysts for hydrogen production. Nature Chemistry. 2016, 8, 179–185. doi: 10.1038/nchem.2416
- Guo, S. et al. Co/CoO Nanoparticles Assembled on Graphene for Electrochemical Reduction of Oxygen, Angewandte Chemie. 2012, 51(47) 11770-11773. doi: 10.1002/anie.201206152
- Raj, B., Van de Voorde, M, & Mahajan, Y. Nanotechnology for Energy Sustainability: Applications of Nanotechnology, John Wiley & Sons, 2017. doi: 10.1002/9783527696109
- Krishna, R. et al. Hydrogen Storage for Energy Application, 2012. IntechOpen.com. doi: 10.5772/51238
- Xin, G. et al. Advanced phase change composite by thermally annealed defect-free graphene for thermal energy storage. ACS Applied Materials & Interfaces. 2014. 6(17) 15262-15271. doi: 10.1021/am503619a
