Most of us are familiar with the concept of “side effects.” This is when something that is designed to be helpful ends up having some harm that goes along with it. For patients with cancer, anti-cancer drugs can be life-savers – literally. The benefits of using these drugs are apparent to anyone whose cancer has gone into remission thanks to their use. It is well known that these drugs can also have some nasty side effects, yet people still choose to use them because the benefits of being cured outweigh the problems (and of course researchers continue to look for ways to reduce those negative effects). Medicine isn’t the only area where this happens: there are many technologies that have lots of benefits to their use, but can come with some potentially bad side effects. Nanoparticles are one example; they have many amazing uses for consumer products, but they can sometimes have harmful impacts on environmental organisms. One goal of the Center for Sustainable Nanotechnology is to try to find options for designing nanoparticles so that those harmful impacts can be reduced.
One important step toward the goal of designing more environmentally friendly nanoparticles is to figure out how different nanoparticles interact with different organisms in the environment. In order to do this, we investigate the interactions at a molecular level. The goal is to be able to predict interactions and any potential toxicity of nanoparticles with organisms, and then use that knowledge to design nanoparticles in a way that will mitigate environmental risk.
As you can imagine, this is a big goal, with a lot of research to keep track of! A group of researchers in the CSN recently published a paper whose purpose was to summarize what is known about how nanoparticle toxicity works, and to describe ways that nanoparticles could be redesigned to reduce environmental side effects.1 The three main nanoparticle toxicity mechanisms are:
- nanoparticles binding to the outside of a cell
- nanoparticles dissolving to release toxic ions
- nanoparticles generating harmful reactive oxygen species (ROS)
Since the potential toxicity of a nanoparticle depends both on the nanoparticle type as well as the organism it is interacting with, each of these three types of toxicity can be important in different situations. When redesigning a nanoparticle, scientists first have to identify the main toxicity mechanism, because strategies for reducing toxicity of course will depend on the source of the toxicity. There has been some research done on redesigning nanoparticles, but we hope our paper will inspire more scientists to assess some of the redesign strategies with different nanoparticle models to be able to make better predictions about what will work. Figure 2 gives a quick overview of the multitude of redesign strategies that we talk about in the paper.
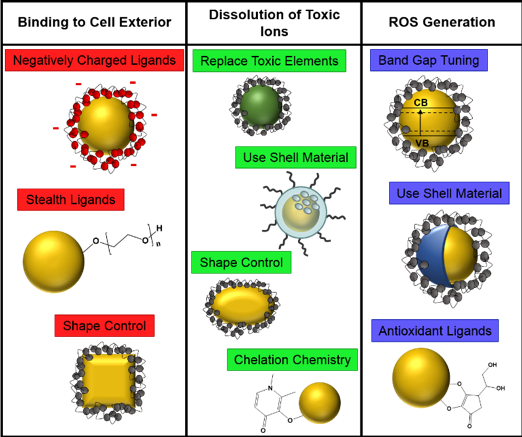
Binding to cell exterior
Nanoparticles sticking to the surface of a cell can disrupt the cell’s membrane, which can kill the cell. Depending on the type of cell, nanoparticles can also enter the cell after binding and potentially cause harm that way. There are several options for redesigning a nanoparticle to reduce its ability to bind. One involves changing the nanoparticle’s surface charge. We know that most cells have a negative charge at their cell surface, and we know that like-repels-like (just like with magnets). So, if nanoparticles are designed to have negative surface charges, they will be less capable of binding to a cell surface and therefore cannot damage cells due to binding.
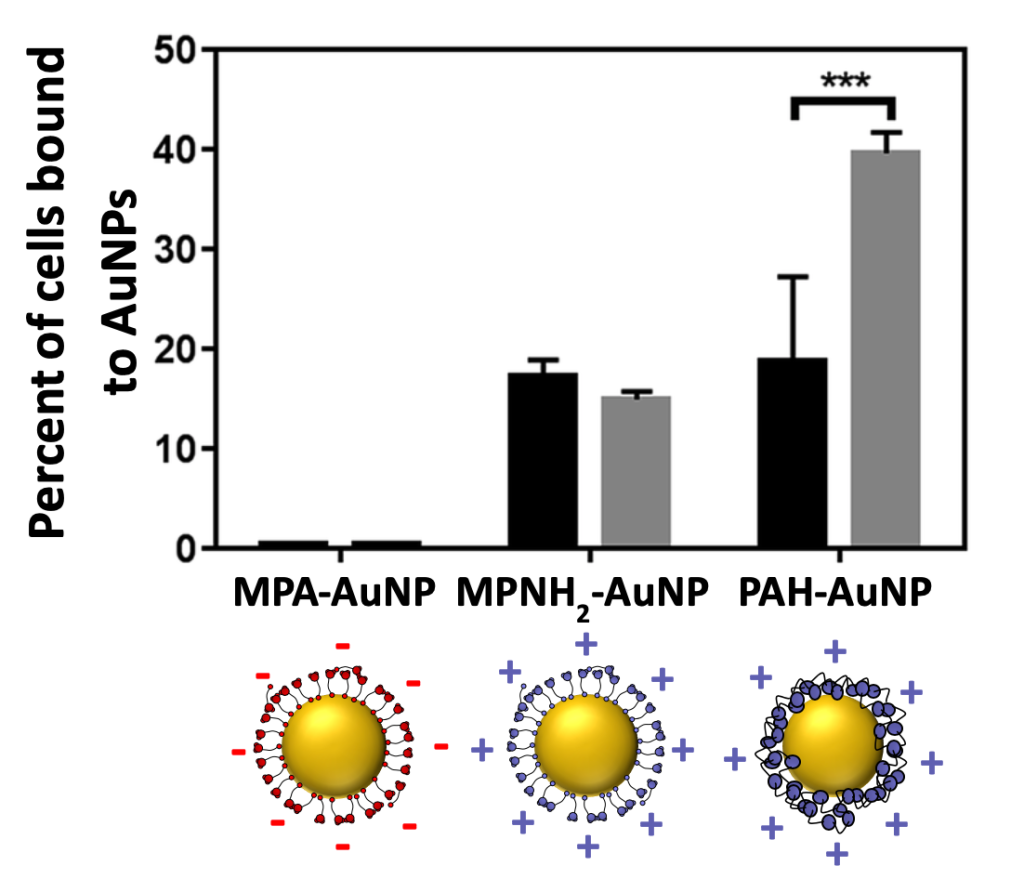
In other CSN work (described in this blog post), we showed that using gold nanoparticles with a positive surface charge led to a lot of binding with the bacterial surface, and subsequently, a lot of toxicity.2 With gold nanoparticles that were made with a negative surface charge, there was hardly any binding observed and the nanoparticles exhibited no toxicity to the bacteria. This shows that being able to redesign a nanoparticle to have a negative charge can reduce its impacts on bacterial cells.
Dissolution
Another way nanoparticles can be toxic to organisms happens when they dissolve in liquid, which releases ions (charged molecules) into the environment. These ions can interact with and be taken up by cells, sometimes with harmful effects. One way to redesign nanoparticles to reduce the release of toxic ions is to replace the elements that are toxic in their ionic form with something less toxic. This approach has been investigated with a model nanoparticle that is often used in the CSN: lithium nickel manganese cobalt oxide (NMC). As we discussed in this blog post and this podcast episode, we know that NMC is toxic to the bacterium Shewanella oneidensis because it releases nickel and cobalt ions into solution.3 So we tried making NMC materials with an increased content of manganese (therefore reducing the nickel and cobalt content) to see if it would be less toxic.4 To measure the toxicity, we used a technique called respirometry, which looks at how much oxygen is being used by the bacteria. As shown in the graph below, it turned out that after exposure to the manganese-enriched NMC materials (green line), S. oneidensis got back up to the same amount of oxygen use as bacteria that weren’t exposed to nanoparticles (black line) more quickly than those exposed to NMC with more nickel and cobalt (blue and red lines).

Reactive Oxygen Species (ROS)
Another way in which nanoparticles can be toxic is in their ability to produce reactive oxygen species (ROS). As the name implies, ROS are highly reactive forms of oxygen (briefly described here), which means they will interact with other materials very easily. They are so reactive that they can react with many different important cell components, such as lipids in the cell membrane, various proteins, and DNA. To reduce how much ROS they generate, nanoparticles can be coated with a shell made of a material that is less prone to ROS production. For example, scientists in one study showed that adding a zinc sulfide shell to cadmium selenide quantum dots reduced their production of ROS without changing the functionality of the quantum dots.5 This demonstrates that coating can be an effective method of reducing impacts on organisms due to ROS generation.

These are just a few examples of the many ways that nanoparticles can be redesigned to reduce their interactions with (and potential toxicity for) organisms in the environment. There is still a lot of work to do to understand the different ways nanoparticles can potentially be toxic to organisms, and there are studies under way in the CSN to understand these mechanisms further. However, we also have enough knowledge that can now be applied to investigate the various methods by which these side effects can be mitigated, which is a newer area to be explored in the field of nanoparticles. It is important to remember that nanoparticles have many great benefits for different consumer products and medical technologies, but by redesigning them to improve their environmental impact, we can work to make nanoparticle use more sustainable in the long run.
REFERENCES
- Buchman, J. T.; Hudson-Smith, N. V; Landy, K. M.; Haynes, C. L. Understanding Nanoparticle Toxicity Mechanisms To Inform Redesign Strategies To Reduce Environmental Impact. Accounts of Chemical Research, 2019, 52, 1632–1642. doi: doi.org/10.1021/acs.accounts.9b00053
- Feng, Z. V.; Gunsolus, I. L.; Qiu, T. A.; Hurley, K. R.; Nyberg, L. H.; Frew, H.; Johnson, K. P.; Vartanian, A. M.; Jacob, L. M.; Lohse, S. E.; Torelli, M. D.; Hamers, R. J.; Murphy, C. J.; Haynes, C. L. Impacts of Gold Nanoparticle Charge and Ligand Type on Surface Binding and Toxicity to Gram-Negative and Gram-Positive Bacteria. Chemical Science, 2015, 6, 5186–5196. doi: 10.1039/C5SC00792E
- Hang, M. N.; Gunsolus, I. L.; Wayland, H.; Melby, E. S.; Mensch, A. C.; Hurley, K. R.; Pedersen, J. A.; Haynes, C. L.; Hamers, R. J. Impact of Nanoscale Lithium Nickel Manganese Cobalt Oxide (NMC) on the Bacterium Shewanella Oneidensis MR-1. Chemistry of Materials, 2016,28, 1092–1100. doi: https://doi.org/10.1021/acs.chemmater.5b04505
- Gunsolus, I. L.; Hang, M. N.; Hudson-Smith, N. V; Buchman, J. T.; Bennett, J. W.; Conroy, D.; Mason, S. E.; Hamers, R. J.; Haynes, C. L. Influence of Nickel Manganese Cobalt Oxide Nanoparticle Composition on Toxicity toward Shewanella Oneidensis MR-1: Redesigning for Reduced Biological Impact. Environmental Sciece: Nano, 2017, 4, 636–646. doi: 10.1039/C6EN00453A
- Ipe, B. I.; Lehnig, M.; Niemeyer, C. M. On the Generation of Free Radical Species from Quantum Dots. Small, 2005, 1, 706–709. doi: 10.1002/smll.200500105
