LEGO blocks may be one of the simplest but most genius toys ever created, as evidenced by their popularity. Taking such simple building blocks that are modular and being able to create nearly endless different combinations of structures is liberating. On the other hand, it’s also great that LEGO can create a set of instructions for the blocks which will result in builders getting the same outcome almost 100% of the time. Wouldn’t it be amazing if there was a way to apply concepts like this to chemistry, using modular building blocks that could both open new possibilities for chemical structures AND allow for following a set of instructions to get a desired outcome almost every time? Well, it turns out that chemists have been doing something very similar to this with a tool called click chemistry.

Over the past couple of decades, chemists have used click chemistry, and a set of guidelines that go with it, to get chemistry to the kind of freedom that LEGO gives to builders. Click chemistry as a paradigm was established around the turn of the 21st century by Hartmuth Kolb, M.G. Finn, and K. Barry Sharpless[1] in an attempt to completely change how chemists used organic synthesis to develop useful new compounds (substances made of more than one kind of atom). They were frustrated by the difficulty of creating connections between carbon atoms. This is something that is necessary for making new compounds, and that nature does very well but is actually quite labor intensive in the lab.
In a 2003 paper, Kolb and Sharpless described that “a click reaction must be of wide scope, giving consistently high yields with a variety of starting materials. It must be easy to perform, be insensitive to oxygen or water, and use only readily available reagents. Reaction work-up and product isolation must be simple, without requiring chromatographic purification.”[2] Not many reactions fit all of those criteria!

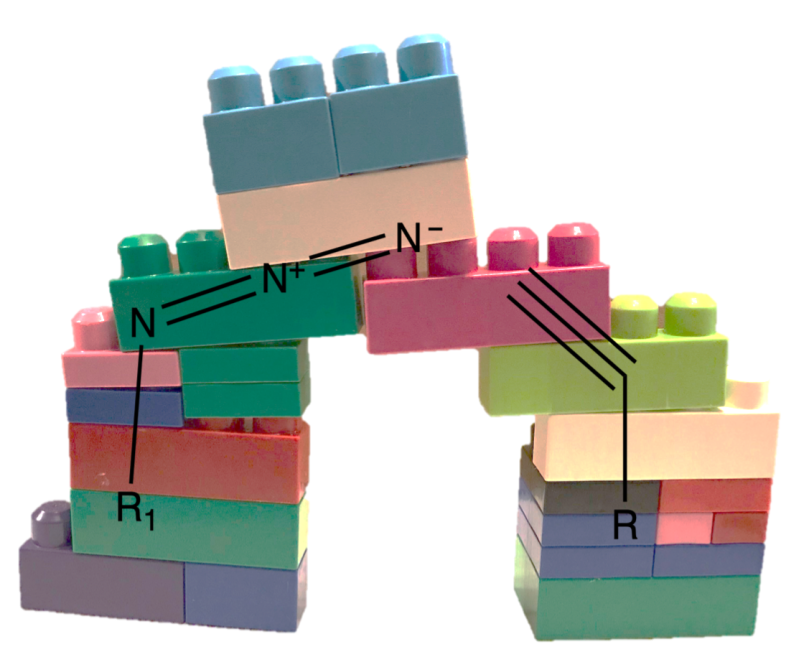
Since then click chemistry has had effects throughout different areas of chemistry. A few of the main click chemistry reactions are now used very widely because they allow a LEGO-like ease of combining different chemical substances together. One big goal was to move toward reactions that could create huge libraries of compounds easily. The libraries would predict when molecules of different shapes could “click” together to make new compounds, like the bumps on one LEGO brick can slot into the holes of another (except the new compounds can’t be pulled apart as easily as LEGOs!). Scientists can then use these libraries to search for compounds that have specific properties, such as being potentially useful as new drug molecules.
This is all exciting, but how can click chemistry contribute to the goals of the Center for Sustainable Nanotechnology?
Click chemistry at its root is about green chemistry; that is, reducing the environmental consequences of working with potentially harmful materials in lab. Examples of green chemistry include finding ways to replace toxic solvents, or improving the efficiency of reactions to reduce waste. In addition to this aspect of sustainability, click chemistry aligns with the research of the Center for Sustainable Nanotechnology in several other ways. For example, consider the many types of nanoparticles in use in the CSN: there are quantum dots, metal oxides, gold and silver nanoparticles, carbon dots, etc. Much of the research in the center involves assessing how these nanoparticles may impact the environment, an important aspect of sustainability because of how common nanoparticles are becoming in industrial and consumer settings. In many cases the chemical properties of these nanoparticles depend on a surface coating that gives them some kind of specific function. In the case of carbon dots, for example, they can be coated with two different molecules to make the surface positively or negatively charged.[4] If adding nanoparticle coatings could be made simple by using click chemistry reagents, huge libraries of nanoparticles could be assembled in the lab to test their biological and environmental impacts.
Just like you can use the same sizes and shapes of LEGO bricks in different combinations to build a huge range creations, the same click chemistry could be used over and over again but with different groups of molecules to easily make new nanoparticle coatings. Being able to quickly and efficiently create a wide variety of surface modifications would make it much easier to monitor how each modification affects organisms differently.[5]
Even when click chemistry isn’t involved specifically in the modification of nanoparticles, it can be useful to monitor the effects of nanoparticles in biological systems. For example, scientists can use click chemistry to help identify proteins that have been “tagged” with special marker molecules that can undergo click chemistry. This is important for distinguishing the proteins that have been tagged from all the other molecules in a sample, which is helpful for seeing how the cells respond to nanoparticles in the environment. As you can imagine, that can be a difficult task. One of the important characteristics of click chemistry is that its reactions can occur in living cells without completely disrupting normal function because bacteria don’t have any of the “LEGO”-like molecules of the click reaction.[7] Almost any kind of different “LEGO” structure (functional groups) can be put on the probe to find it once it interacts with the protein.
Click chemistry has made its way into the field of nanotechnology, and in doing so has given nanotechnology an implicitly sustainable outlook. Because of its emphasis on reactions that occur as easily as LEGO bricks snapping together in very mild reaction conditions while producing minimal toxic byproducts, click chemistry and sustainable nanotechnology go hand in hand.
EDUCATIONAL RESOURCES
- LEGO Group & MIT Edgerton Center: LEGO Atoms and Molecules: Chemical Reactions Teacher’s Guide
- Oak Ridge National Laboratory Eugene P. Wigner Distinguished Lecture Series in Science, Technology and Policy: Video of Nobel Laureate K. Barry Sharpless lecture on “New Developments in Click Chemistry“
REFERENCES
- Kolb, H.C., Finn, M.G., & Sharpless, K.B. Click chemistry: Diverse Chemical Function from a few good reactions. Angewandte Chemie, 2001, 40(11) 2004-2021. doi: <a href="https://dx.doi.org/10.1002/1521-3773(20010601)40:1110.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5
- Kolb, H.C. & Sharpless, K. B. The growing impact of click chemistry on drug discovery. Drug Discovery Today, 2003, 8 (24), 1128-1137. doi: 10.1016/S1359-6446(03)02933-7
- Liang, A. The copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview. Coordination Chemistry Reviews, 2011, 255 (23–24), 2933-2945. doi: 10.1016/j.ccr.2011.06.028
- Yao, B. et al. Carbon Dots: A Small Conundrum. Trends in Chemistry, 2019, 1 (2), 235-246. doi: 10.1016/j.trechm.2019.02.003
- Yi, G. et al. Application of click chemistry in nanoparticle modification and its targeted delivery. Biomaterials Research 2018, 22 (1), 13. doi: 10.1186/s40824-018-0123-0
- Jung, Y. K. et al. Cell Nucleus-Targeting Zwitterionic Carbon Dots. Scientific Reports. 2015, 5, 18807. doi: 10.1038/srep18807
- McKay, C. S. & Finn, M. G., Click Chemistry in Complex Mixtures: Bioorthogonal Bioconjugation. Chemical Biology, 2014, 21 (9), 1075-1101. doi: 10.1016/j.chembiol.2014.09.002
DISCLOSURE: The CSN, Sustainable-Nano.com, and the author have not received any compensation for writing this post. We have no material connection to LEGO or any products mentioned.
