What is the color of “typical” carbons? As black as charcoal, or as clear as diamond (Fig. 1)? Can we imagine any other answers, such as blue, green, or red? You may think I am crazy, but actually, the answer is “Yes”!

To explain the idea of multi-colored carbon, I’m going to tell you about carbon dots. Recently, I was the lead author on a collaborative article from the Center for Sustainable Nanotechnology (CSN) in the journal Carbon, titled “Investigation of phosphorous doping effects on polymeric carbon dots: Fluorescence, photostability, and environmental impact.”1 So, you may wonder: what are carbon dots? How do they fluoresce? And are they toxic? Let me walk you through these questions one by one.
What are carbon dots?
Basically, carbon dots are nanoparticles consisting primarily of carbon with small amounts of nitrogen and oxygen. Within the CSN, we synthesize these particles using a so-called “bottom-up” method, namely, starting with molecular precursors such as citric acid or malic acid and ethylamine and building up to nanoscale particles (Fig.2).
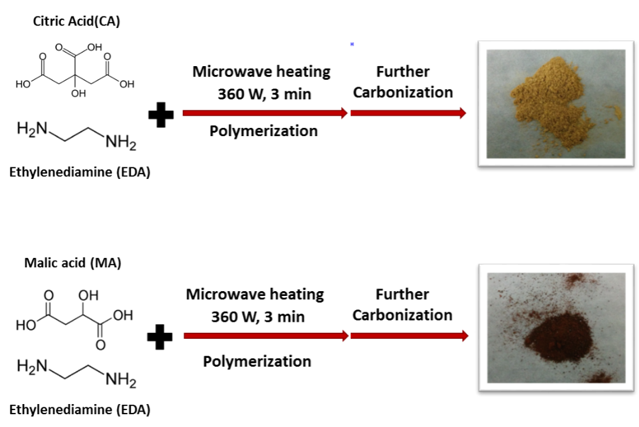
Precursors refer to starting chemicals we used to initiate reactions. They are somewhat analogous to ingredients when you are baking or cooking: eggs, flour, and sugar would be precursors to cake. The options for carbon precursors can be very broad, from laboratory chemicals (e.g., small organic acids like citric acid, malic acid, or aspartic acid) to natural products (e.g., biomass, plants, or even fruits).2 We chose citric acid and malic acid for our paper because they are environmentally benign, naturally occurring chemicals and they are usually used as food additives. Citric acid might sound familiar because it can be found in a variety of fruits and vegetables, such as lemons and limes; malic acid is the main acid in many fruits like grapes and peaches.
Another lesson we can learn from cooking has to do with heat. If you spend any time cooking, you know that heat is usually an essential component for making ingredients turn into the final product you want to eat. Making carbon dots is no different: by taking advantage of microwave-assisted heating, carbon dots can be prepared in less than five minutes. We used to use a microwave like the one you’d find in your kitchen to make carbon dots, but we made the change to a microwave for science research. To be more specific, reaction temperature and pressure can be well controlled inside a research microwave, and more importantly, the microwave radiation is distributed in a homogenous manner that is beneficial for stable reactions, rather than a chaotic manner like in a typical kitchen microwave. When heated in the microwave, both citric and malic acid will react with a small amount of ethylenediamine in aqueous solution and undergo a series of chemical reactions to make carbon dots.
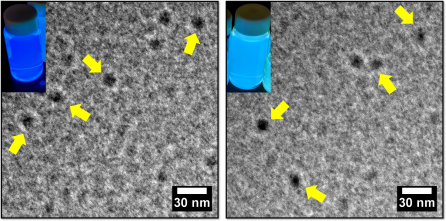
The carbon dots we made have a round shape with a diameter of less than 10 nm, which we determined using transmission electron microscopy (Fig.3).1 (Electron microscopes allow scientists to see things that light microscopes are not able to capture.)
How do carbon dots fluoresce?
The fantastic part about these carbon dots is their photoluminescence. Basically, photoluminescence is a glowing phenomenon from any form of matter after the absorption of light. You have seen plenty of examples of it in your everyday life, which are discussed in detail in this popular blog post by Tom Kuech. Photoluminescence can be roughly divided into two categories, depending on how long the glowing lasts: fluorescence and phosphorescence. If the glow immediately fades after you remove the light source, it is fluorescence; phosphorescence will continue a bit longer with the absence of a light source (Fig.3 shows one great fictional example: glow-in-dark Link!).

As shown in figure 4, another advantage of carbon dots is that they are usually highly soluble in water and can be illuminated with long-wavelength ultraviolet light (UV). The example in these images is 365nm light which is in the range of ultraviolet wavelengths from the sun. Having two different precursors (citric acid vs. malic acid), a solution of carbon dots in water will emit different colors. For instance, the citric acid carbon dots will emit a blue color while malic acid carbon dots emit a teal color.
Another thing we have learned about carbon dots is that, after a careful purification procedure where we remove non-fluorescent components and residues, carbon dots can be separated into more colorful components, covering the entire visible light range (Fig.5).

So, why are these carbon dots photoluminescent? Currently, the mechanism behind carbon dot fluorescence is under hot debate, and several hypotheses have been proposed. Some research has indicated that the particle size of carbon dots is controllable by manipulating some of the synthesis conditions, which is similar to traditional quantum dots where the band gap emission can be tuned.3 However, as there are other elements inside carbon dots, such as nitrogen and oxygen, some other researchers hypothesize that compared to pure carbon networks (like graphene, charcoal, or diamond), bonding between these atoms and carbon atoms will influence the internal structure and surface chemistry of carbon dots, leading to the fluorescence emission.3
Another popular explanation is that there are specific molecules formed during the carbon dot synthesis that contribute to the emission behavior, especially for citric acid-based carbon dots.4 So the question of why exactly carbon dots fluoresce is still unanswered. But even without the complete picture, we scientists can still explore how they may impact the environment.
Are carbon dots toxic or not?
Carbon dots are generally considered to be a “green” alternative to traditional quantum dots, so for our article we tested their environmental impact using a bacterium called Shewanella oneidensisMR-1 as a model microorganism. Bacteria are often used in studies like this because they are ubiquitous in the environment and play important roles in the ecosystem, such as contributing to the geochemical nutrient cycling. We have also used this specific type of bacteria in some of our previous work. In terms of predicting toxicity, if the growth of bacteria is suppressed, then it is possible that higher-order organisms will be impacted as well. One way to know if the health of our bacterial samples is compromised is to evaluate their growth. Healthy bacterial colonies appear pale yellow or yellow (Fig.6 right) and dying colonies are deep yellow or orange.
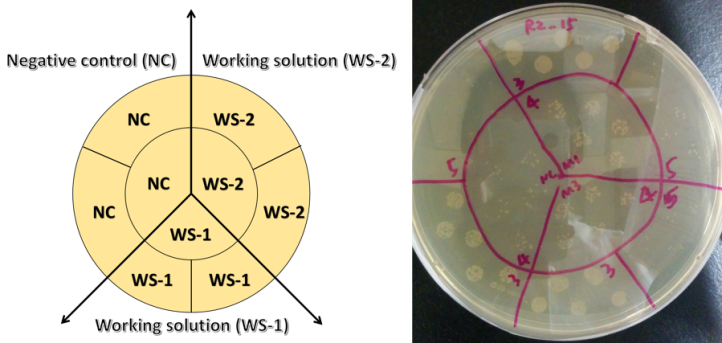
If bacteria are living in a “toxic environment”, they will have trouble growing and as such they will form fewer colonies than usual. The technique used to assess bacterial toxicity is called a “colony counting assay” (Fig.6), and basically, we are counting the number of colonies formed after exposing the bacteria to carbon dots. We then do a statistical comparison to a negative control where bacteria were not exposed to any carbon dots. As shown in Fig.6, it is clear that after being exposed to carbon dots, bacteria formed just as many colonies as the negative control group, even at higher nanoparticle concentrations. With these data, we showed that for bacteria growth, the influence caused by carbon dot exposure is negligible. In other words, carbon dots are environmentally benign at least as far as Shewanella is concerned.
The take-home message for our paper is that carbon dots can be cheaply and quickly prepared, and the final products can fluoresce in colorful ways. More importantly, compared to traditional quantum dots, they are not toxic to the bacteria that we studied.5
EDUCATIONAL RESOURCES
- Carbon Quantum Dots: A Safe Tool to Learn about Quantum Phenomenon in Nanomaterials: classroom activity for making your own carbon dots by Cruz et al. in Journal of Laboratory Chemical Education, 2017. DOI: 10.5923/j.jlce.20170503.02
- Carbon dots: a modular activity to teach fluorescence and nanotechnology at multiple levels. by Pham, et al. in Journal of Chemical Education, 2017. DOI: 10.1021/acs.jchemed.6b00995
REFERENCES
- Zhi, B.; Gallagher, M. J.; Frank, B. P.; Lyons, T. Y.; Qiu, T. A.; Da, J.; Mensch, A. C.; Hamers, R. J.; Rosenzweig, Z.; Fairbrother, D. H.; Haynes, C. L., Investigation of Phosphorous Doping Effects on Polymeric Carbon Dots: Fluorescence, Photostability, and Environmental Impact. Carbon 2018, 129, 438-449. DOI: 10.1016/j.carbon.2017.12.004
- Zhang, X.; Jiang, M.; Niu, N.; Chen, Z.; Li, S.; Liu, S.; Li, J., Natural-Product-Derived Carbon Dots: From Natural Products to Functional Materials. ChemSusChem 2017, 11, 11-24. DOI: 10.1002/cssc.201701847
- Xiao, L.; Sun, H., Novel Properties and Applications of Carbon Nano-Dots. Nanoscale Horizons 2018. DOI: 10.1039/C8NH00106E
- Shi, L.; Yang, J. H.; Zeng, H. B.; Chen, Y. M.; Yang, S. C.; Wu, C.; Zeng, H.; Yoshihito, O.; Zhang, Q., Carbon Dots with High Fluorescence Quantum Yield: The Fluorescence Originates from Organic Fluorophores. Nanoscale 2016, 8, 14374-14378. DOI: 10.1039/C6NR00451B
- Williams, D. N.; Pramanik, S.; Brown, R. P.; Zhi, B.; McIntire, E.; Hudson-Smith, N. V.; Haynes, C. L.; Rosenzweig, Z., Adverse Interactions of Luminescent Semiconductor Quantum Dots with Liposomes and Shewanella Oneidensis. ACS Applied Nano Materials 2018, 1, 4788-4800. DOI: 10.1021/acsanm.8b01000
MORE ABOUT COLONY COUNTING
What’s in a healthy colony?
Rather than counting the number of real bacteria, we only count the number of colonies. That’s why in microbiology, we have a unit called CFU (colony forming unit). In addition, during the lag phase (the beginning of bacteria growth), bacteria get themselves adapted to the environment, so in this phase, the growth is quite slow. So, we don’t measure the growth speed. For more, see this Wikipedia article and this blog post.
