Yes folks, we’ve done it. You probably didn’t think we could, but we found another way to talk about beer and nanoparticles!
Have you ever pondered why bubbles form and rise from the bottom of a beer glass? There is an astonishing amount of research on the bubbling or “fizzics” of beer. During the production of beer, carbon dioxide (CO2) is naturally produced along with ethyl alcohol (the type of alcohol we drink) as the yeast consumes sugar and additional CO2 is added during packaging to keep the beer fresh and zingy. But, what is the process that allows the bubbles to form at the bottom of the glass?

This process is called nucleation. For beer, nucleation is the process in which a small defect in the glass acts as the starting point for the formation of a CO2 bubble. In fact, many beer glasses and champagne flutes are nucleated, meaning they are purposefully etched, so that the dissolved CO2 in the beer has a place to gather and form larger bubbles.1 Once the bubble grows too large and buoyant to stay at the bottom of the glass, it detaches from the glassware, rises through the beer, and continues growing and gathering CO2 along the way. Having a bubbly beer and the foam head at the top is important when drinking the beer because it helps you appreciate the aroma, which is a major component of flavor and helps you distinguish the flavors of beer. So, nucleation is important for your overall beer experience! If you’re interested in learning more, check out this nice, brief report on how chemist Richard Zare started thinking about beer bubbles.
Before we consider how nucleation relates to nanoparticles, let’s consider a different example of nucleation for those who aren’t 21 yet. You may recall a string of videos going around of folks putting a water bottle in their freezer, striking the bottle, and seeing the instant formation of ice.
The formation of ice also begins with the process of nucleation. In this dramatic example, water has cooled below its freezing point (0 °C) but won’t begin the freezing process until it encounters a nucleation site. This can be caused by an imperfection of the container, the addition of an object (like a stick or salt), or a physical disruption, like a shock wave from striking the container. With a shock wave, the molecules of water move so abruptly that they end up pulling away from each other, creating a cavity or bubble in the water. That bubble is enough disruption to stimulate the formation of ice crystals, which then cascade throughout the container.2
Here is a video of me striking a plastic tube of super-cooled water (-20 °C or -4 °F):
If we pick a frame to look at right after the shock wave, you can see a couple areas where the nucleation process began:

So how does all of this talk of nucleation relate back to nanoparticles, you ask? Well, to answer this we first need to think about how nanoparticles are synthesized. There are two methods of nanoparticles synthesis: top-down or bottom-up. The top-down approach involves a large structure being broken up into nano-sized units, whereas the bottom-up approach involves chemical reactions that lead to the aggregation, or clumping, of atoms into particles.
Much like how the CO2 bubbles in beer form because of nucleation, the bottom-up method to synthesize nanoparticles also begins with nucleation. When synthesizing metal nanoparticles, there are generally three ingredients: a metal salt, a reducing agent, and a stabilizing agent. The first step of nanoparticle synthesis is reducing a metal salt into a single atom of pure metal. For example, with gold nanoparticles the gold salt (in which gold is positively charged) is reduced (gains electrons to become more negative) by the reducing agent to become pure gold (neutrally charged). These gold atoms become sites of nucleation, meaning that each atom will act as a starting place for crystal growth for the rest of the nanoparticle, just like the congregation of CO2 from beer.
In the second step of nanoparticle synthesis, the stabilizing agent serves as a way to stop the growth of the nanoparticles. Otherwise, the nanoparticles will keep growing based on the availability of the metal atoms in the solution, just like how the ice keeps forming until there is no more available water. That unchecked growth would lead to particles that weren’t nano anymore, just normal bulk-sized gold. Instead, the formation of nanoparticles happens at numerous locations at different nucleation sites (gold atoms) so we end up with many gold nanoparticles in solution.
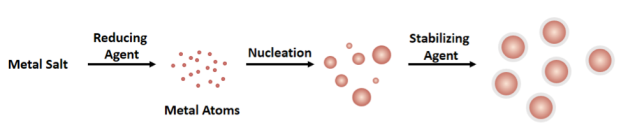
Controlling nucleation and growth of nanoparticles is very important for chemists so that we can try to control the size and shapes of the nanoparticles we synthesize. Nanoparticles are used in a variety of technologies, and it is often very important that they are all consistent in size and shape to ensure they are effective. For example, changes in nanoparticle shape can change their ability to be taken up by the body in medical applications.3 Although this nucleation and growth process is so fundamental, scientists still don’t have a single theory or model that fully predicts or describes how nanoparticles form a particular size and shape.4 If you’re interested in investigating different types of nucleation and theories of nanoparticle growth you should also check out the 2014 review by Thanh et al.5
The video above shows my lab mate Emily synthesizing some gold nanoparticles. Here she has a solution of gold salt (HAuCl4) to which she adds citrate, which acts as both the reducing and capping agent. She can control the size of the nanoparticles by changing the ratio of gold to citrate. If you’re interested in why the gold nanoparticles are red, check out this blog post!
The formation of nanoparticles is one of the smallest scale examples of nucleation, but the process of nucleation is also used in a variety of other fields, from cloud seeding to culturing pearls. As it turns out, nucleation is also one of the important reason the Mentos and Diet Coke experiment causes a fizz geyser! Enjoy all your favorite forms of nucleation.



Cheers!


EDUCATIONAL RESOURCES
- National Association of Geoscience Teachers: Mentos and soda eruptions- lessons on explosive volcanic eruptions
- Rowat, et al. The Science of Chocolate: Interactive Activities on Phase Transitions, Emulsification, and Nucleation, Journal of Chemical Education (may require subscription; summary here)
REFERENCES
- Shafer, N. E., Zare, R. N. (1991). Through a Beer Glass Darkly. Physics Today, 44(10), 48. doi: 10.1063/1.881294
- Gitlin, S. N., Lin, S.-S. (1969). Dynamic Nucleation of the Ice Phase in Supercooled Water. Journal of Applied Physics, 40(12), 4761- doi: 10.1063/1.1657285
- Banerjee, A., Qi, J., Gogoi, R., Wong, J., Mitragotri, S. (2016). Role of Nanoparticle Size, Shape and Surface Chemistry in Oral Drug Delivery. Journal of Controlled Release, 238, 176- doi: 10.1016/j.jconrel.2016.07.051.
- Polte, J. (2015). Fundamental Growth Principles of Colloidal Metal Nanoparticles – A New Perspective. CrystEngComm, 17, 6809- doi: 10.1039/C5CE01014D
- Thanh, N. T. K., Maclean, N., Mahiddine, S. (2014). Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chemical Reviews, 114, 7610-7630. doi: 10.1021/cr400544s

Thanks for the references. There is always more to study on this topic. The nucleation is an interesting topic. I didn’t really think about that in this detail. Thank you very much for this blog post. The interesting part for me was about the diet Coke and Mentos.