Have you ever heard the phrase “walking on eggshells”? The phrase is usually meant to convey the message that you should take care in not disrupting or offending someone. It turns out, though, that you can actually walk on eggshells without breaking them. This is achievable because the person’s weight is equally distributed over the eggs so as not to apply too much pressure to any individual egg, which in scientific terms is called your mass density (mass per unit area). I like to use mass density as a concept to help explain the idea of charge density. While mass density has to do with spreading weight across a surface, charge density means the amount of charge spread across an area.

Charge density is an important factor in the development of new nanotechnologies but one of the most relevant for this discussion is in the development of antimicrobial surfaces. All of these applications can make use of molecules called polymers and oligomers (which I will talk more about in a bit) to achieve specific properties. If we look at polymers in antimicrobial films for instance, some researchers have found that there is a critical charge density threshold over which a polymer film demonstrates optimal antimicrobial efficiency. Others have also found that the anti-bacterial properties can be tuned simply by changing one key group of atoms present in the antimicrobial films. In fact, one of our previously posted public-friendly article summaries discusses just that!
These factors, and this ability to tune the property of films (or maybe even nanoparticles functionalized with different polymers) could allow us to better understand and develop nanomaterials that can effectively target different types of cells while leaving other cells unaffected. In a pair of recent articles I wrote with colleagues from the Center for Sustainable Nanotechnology, our approach involved using a suite of positively-charged polymers and model biological membrane mimics called supported lipid bilayers.1,2 (For a refresher on what supported lipid bilayers are and why we use them, check out this post from Lisa Jacobs.)

In the two recently-published research papers, my co-authors and I wanted to understand some toxic effects that people have observed when cells are exposed to nanoparticles wrapped in coating molecules. Here’s an analogy: Imagine that you try a chocolate from one of those boxes with an adventurous assortment of fillings. While you initially enjoy the unfamiliar flavor, you later realize that you have developed an allergic reaction! While trying not to scratch that benign but annoying rash, you wonder whether it was the filling that made you sick, or (one of my worst fears) are you developing an allergy to chocolate? Or maybe there is something about the combination of the two? How can you figure out which made you sick?
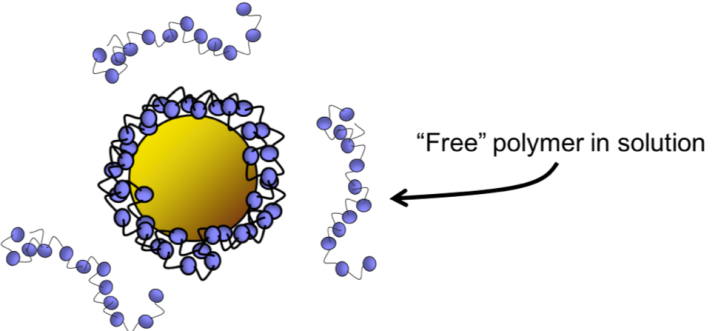
One way would be to try out the chocolate coating by itself, separate from the filling, to see if it caused the same reaction. In our study of wrapped nanoparticles, we wanted to know if the observations of toxicity are a result of the nanoparticle, the coating, or both. In order to get a better picture, we focused our studies on understanding the potential adverse effects of the coatings on their own. To do this, we did experiments to study how the wrapping molecules, called polymers and oligomers, interact with model biological surfaces.
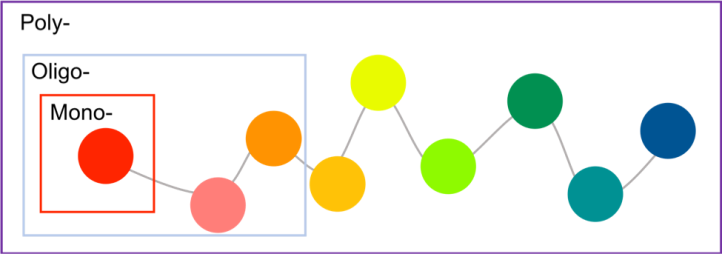
But what are polymers and oligomers? The word “polymer” is derived from “poly-” meaning many and “-mers” meaning parts. Polymers are composed of many monomers (where “mono-“ means one). Oligomers are similar to polymers in that they are made from a collection of individual monomer groups, but oligomers tend to have only a few repeat units (“oligo–“ means few) (Figure 4). We chose various types of positively-charged polymers for this project because of the diversity of their charged groups, and because of previous research studies which showed that they can have significantly different interactions with biological membranes than nanoparticles by themselves (Figure 3). (For a cool polymer-related superhero, check out this video from the middle school winner of the Generation Nano 2018 contest).
All Charged Up!
In order to quantify the charge density of the polymers/oligomers, we used spectroscopy (which deals with the way that light interacts with matter) and very sensitive mass balances including quartz crystal microbalance with dissipation monitoring (QCM-D), to estimate the charge density and the amount of polymer adsorbed to the membrane surface. Like most biological surfaces, our supported lipid bilayers carry a negative charge that is distributed over a given area and is thus associated with a given charge density (remember that charge over area is charge density).
What did we expect to happen when we allowed polymers to interact with the surfaces of our model cell surfaces? Well, you are probably familiar with the concept that opposites attract and here we expected that the positively charged polymers would be attracted to the surface of lipid bilayer. (See the video about Peppy T. Polymer for an example of adsorption of the polymer to the cellular membrane of gram negative bacteria.) Sure enough, we found that the polymers and oligomers adsorb to the surface of our bilayers, and in some cases the charge of the surface can be neutralized (the overall charge is zero) or reversed (the initially negatively charged surface can become positively charged).
What surprised us was that it turns out that all of the polymers we looked at seemed to “like” the surfaces of our membranes equally. This implies that the chemical structure may not be as important in this system as we thought (at least under these conditions). This was a surprise because some other studies have found that the identity of the functional group can change biological outcomes in real biological systems. These results are important because they have provided some information that will be useful in helping us to better understand how some polymers (or even nanoparticles coated with different polymers) might cause toxic effects in real systems. As far as our analogy about chocolate coated candies, the mystery remains somewhat unsolved. Stay tuned for more work on this topic!

REFERENCES
- McGeachy, A., Caudill, E., Liang, D., Cui, Q., Pedersen, J., & Geiger, F. (2018). Counting charges on membrane-bound peptides. Chemical Science, 9(18), 4285-4298. doi: 10.1039/C8SC00804C
- McGeachy, A., Dalchand, N., Caudill, E., Li, T., Doğangün, M., Olenick, L., Chang, H., Pedersen, J., & Geiger, F. (2018). Interfacial electrostatics of poly (vinylamine hydrochloride), poly (diallyldimethylammonium chloride), poly-l-lysine, and poly-l-arginine interacting with lipid bilayers. Physical Chemistry Chemical Physics, 20(16), 10846-10856. doi: 10.1039/C7CP07353D
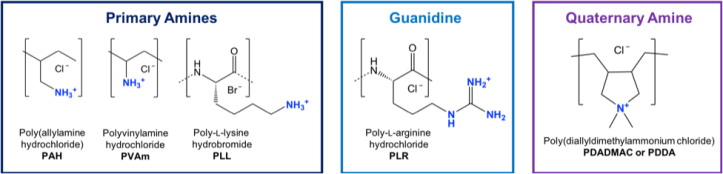
More about Figure 5: Functional Groups and Chemical Illustrations
All of the polymers that we looked at in this work contained cationic (positively charged) groups that includes primary amines (meaning the nitrogen is only connected to one carbon atom), quaternary amines (meaning the nitrogen is connected to 4 carbon atoms), and guanidine. Each of these chemical functionalities can have unique ways of behaving and interacting with other functional groups.
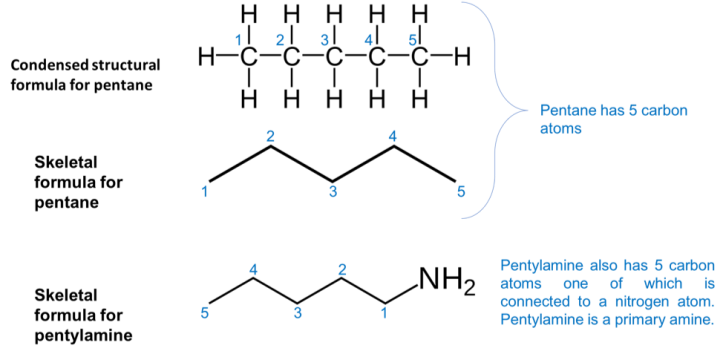
Are you curious about how the chemical structures are presented? Scientists use this sort of short hand notation, sometimes referred to as a skeletal chemical structure, to illustrate the chemical structure. In this notation, each line is a bond between two atoms and each of the vertices or terminal groups is a carbon atom. To clarify the illustration, all of the hydrogen atoms that are bonded to the carbon atom are omitted and only non-carbon atoms are explicitly shown. See an example below: