The United States celebrated Independence Day last week, and most people in the US probably managed to see some fireworks. Besides the fireworks, one of my favorite things this time of year is another kind of light show—fireflies! There’s something almost ethereal about fireflies in a field on a calm summer night. When I was a kid, I loved catching them and putting them in a jar. I’d poke holes in the lid and watch the fireflies for a while as they crawled up the side and flew around, and then I would release them again. I always wondered how their lanterns glow, and how they’re so bright that you can see them across a big field.

It turns out that the light source in firefly lanterns is a chemical reaction in which an organic molecule called luciferin reacts with adenosine triphosphate (an energy source in organisms), the luciferase enzyme, and oxygen to form a different chemical called oxyluciferin.1 The reaction equation looks like this:
luciferin + ATP + luciferase + O2 → oxyluciferin* + AMP + CO2 + luciferase
Notice how the luciferase is on both sides of the equation? That’s because it’s a catalyst—it speeds up the reaction, but is not used up by the process. AMP (adenosine monophosphate) is what ATP becomes after it is used for energy. The oxyluciferin has an asterisk (*), meaning it has an electron in the excited state, and this is the key to why fireflies glow.
We’ve discussed electron energy states on the blog before, such as in this post. Think of energy levels like rungs on a ladder, though not evenly spaced. The bottom energy level is the ground state, and all the others are higher energy “excited” states. Electrons don’t like to be in an excited state — they prefer to be in the ground state, so they tend to return there whenever they’re put in the excited state.

The energy from the excited state can’t just disappear when the electron makes the jump to the ground state, so the electron releases energy in the form of a packet of light called a photon (see this blog post for more information about photons and light!). And so we have another process we can write out in the form of an equation:
oxyluciferin* → oxyluciferin + light
The more electrons that make this leap and emit a photon, the brighter the light is. So you might think that with this understanding of oxyluciferin, a firefly’s glow is totally explained. But it turns out that the light of a firefly is brighter than we would expect it to be, based on how small fireflies are—they only have so much oxyluciferin! So, what makes their light so bright? In a recent paper,2 researchers found out it’s all about the structure of the firefly lantern. The part of the lantern where the reaction occurs is protected by a cuticle layer, which has a shape that makes the light brighter. Let’s zoom in and take a look!
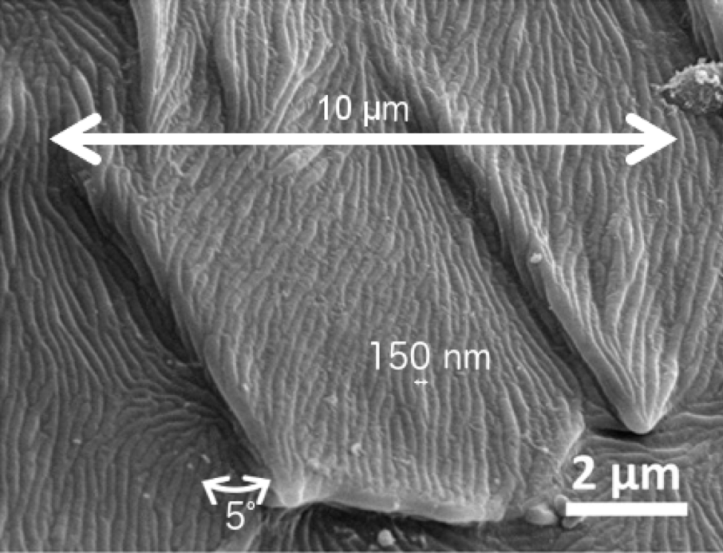
As seen in the microscope image above, the cuticle has tiny overlapping scale-like structures about 10 micrometers (mm) wide—about 1/5 the width of an average human hair—and at a slight incline from the surface, about 5°. Each scale has lengthwise ridges 150 nanometers (nm) wide and 110 nm deep—about 400 times smaller than the width of a hair! These structures bend the light so that more of it can be seen from more viewing angles than if the cuticle were a smooth layer—kind of like tiny magnifying glasses—making the light brighter. Fireflies use these lights to communicate with each other, so the brighter light allows them to metaphorically shout to each other across the field.
This structure of the firefly cuticle is kind of like the ridged shape of the lens on a lighthouse light, although lighthouse lenses are also designed to focus the light in a certain direction. These lenses are called Fresnel lenses, after the French scientist who developed them in the early 1800s.3

So, that’s great for the fireflies and lighthouses, but can this help the rest of us? The answer is yes, because of one phrase that we’re seeing everywhere: energy efficiency. The more energy-efficient something is, the more the energy put in is converted into its intended purpose. Take a lightbulb, for example: we want the lightbulb to produce light, but not energy we can’t use or don’t want, like the heat from an incandescent bulb. When energy conversion is more efficient, and when the light source is brighter, it takes less energy to have the same brightness as a less efficient bulb.
You’ve probably heard about light emitting diodes (LEDs), those energy-efficient lightbulbs that last much longer than incandescent bulbs. LEDs don’t produce anywhere near as much heat as incandescent bulbs do, and so more of the energy used goes into the production of light; hence, they are more energy-efficient. Organic LEDs (OLEDs) use organic molecules, and can be even more energy-efficient than other types of LEDs. They’re also thinner and more flexible. OLEDs are currently used in the displays of phones and digital cameras, but their flexibility could even make them usable for foldable devices in the future!4

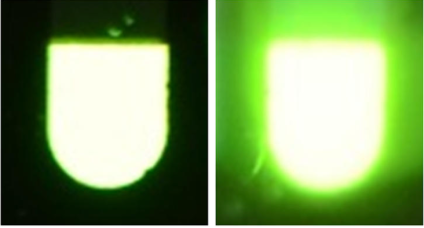
With the motivation for brighter, more energy-efficient light sources, scientists have looked to fireflies for inspiration in a design for OLEDs. In the same paper discussed above, the researchers created a mold based on the cuticle structure of fireflies using photolithography (etching small features using light, explained more in this blog post). They had to use photolithography because no physical tool is small enough to cut those grooves. They cast a resin (liquid plastic that later hardens) in this mold to form the outer layer of the OLED. Compared with the same OLED design with a smooth surface, the OLED with the firefly-inspired design was up to 61% brighter!2 (See for yourself below!) Imagine the reduction of our electricity bills if we used lightbulbs inspired by fireflies!
If you’ve never seen a field of fireflies, go see one if you get the chance. Maybe later you’ll remember that they are so bright because of nano-sized structures, but for the moment, just marvel at one of the most beautiful sights of summer.
EDUCATIONAL RESOURCES
- National Science Teachers Association Press: Next Time You See a Firefly by Emily Morgan
- Michigan State University Extension: Teaching children about science with fireflies by Darren Bagley
- Florida State University Science, Optics, & You: Augustin-Jean Fresnel by Michael W. Davidson
REFERENCES
- Branchini, B. R.; Behney, C. E.; Southworth, T. L.; Fontaine, D. M.; Gulick, A. M.; Vinyard, D. J.; Brudvig, G. W., Experimental Support for a Single Electron-Transfer Oxidation Mechanism in Firefly Bioluminescence. Journal of the American Chemical Society 2015,137(24), 7592-7595.
- Kim, J. J.; Lee, J.; Yang, S. P.; Kim, H. G.; Kweon, H. S.; Yoo, S.; Jeong, K. H. Biologically Inspired Organic Light-Emitting Diodes, Nano Lett. 2016, 16, pp. 2994−3000
- Woodford, C. Fresnel Lenses. https://www.explainthatstuff.com/fresnel-lenses.html. Updated December 1, 2017.
- Freudenrich, C. How OLEDs Work. https://electronics.howstuffworks.com/oled.htm (accessed June 1, 2018).
