This year, Pokémon Go is celebrating the Halloween season with extra spooky Pokémon! As an avid fan of Pokémon as well as a chemist, I wondered, “Is there a connection between ghost Pokémon and chemistry?”
With a little inspiration from last week’s Mole Day, I was able to find one. (Mole Day is October 23 because the mole unit in chemistry is 6.02×1023.)
Pokémon Go is a game that allows you to catch creatures called Pokémon in “augmented reality.” A Pokémon is linked to a certain GPS coordinate and appears (on your cell phone) as you near that coordinate. Additionally, each Pokémon has unique features, just like how each animal of a species is unique. When you catch a Pokémon, the game gives you information about the Pokémon’s height and weight and strength. These aspects can vary from Pokémon to Pokémon of the same type.
Today, as I was getting off the bus, I saw and caught a Gastly.

A Gastly is a ghost/poison-type Pokémon so it is a perfect Halloween catch! As the name suggests, Gastly is mostly made up of gas. And, I noticed my particular Gastly weighed 0.09 kg. I found myself wondering, “How many moles of gas are in a Gastly?”
To figure this out, you have to answer two main questions:
- What type of gas is Gastly made of?
- How do I figure out how many moles are in a gas?
Gastly is almost always drawn as a black sphere surrounded by purple gas. The Pokémon franchise indicates that Gastly’s body is mostly made up of a poisonous gas. Based on the color and the properties, I think it is not unlikely that Gastly is made of iodine gas. Iodine is a chemical element and a member of the halogen family. As a solid, it is a purpley-black metallic color and it can sublime (go directly from solid to gas) to a bright purple gas. All of this matches the description of a Gastly!

Additionally, iodine gas is toxic just like Gastly. The lethal dose for an average human is calculated to be approximately 2.4 grams (or 0.0024 kg)1. Given my Gastly has a weight of 0.09 kg, I can conclude that if the body is all iodine, he’s a potentially very deadly Pokémon despite the innocent and playful personality this species is depicted as having in the cartoon.
So, how many moles of gas are in my Gastly? I just need some quick math to figure this out.
Starting with the periodic table, which shows the molar mass of each element underneath its symbol, I know that each iodine atom has an atomic mass of approximately 127 g/mole.
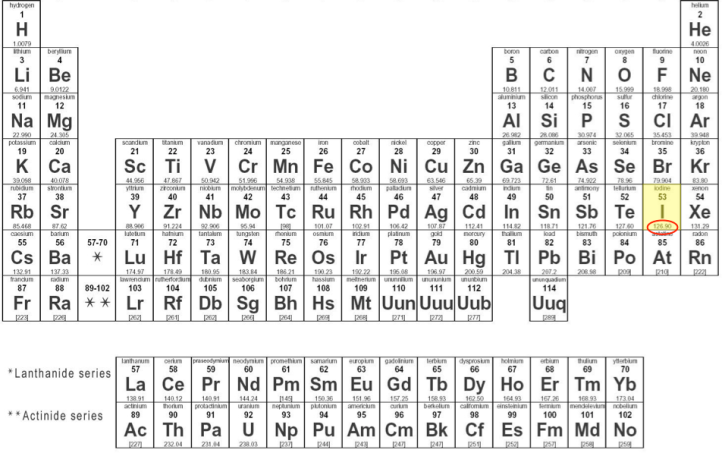
Elemental iodine is a diatomic molecule with the formula I2. Other naturally occurring diatomic elements (elements that make molecules with two atoms) are hydrogen, oxygen, nitrogen, chlorine, bromine, and fluorine. With the atomic mass of 127 g/mole and with two iodine atoms in each iodine molecule, I2 has a molecular mass of approximately 254 g/mole.
So, for a Gastly that has a mass of 0.09 kg,
0.09 kg = 90 grams
(90 grams) x (1 mole/254 grams) = 0.35 moles
We can go further with this and figure out how much space Gastly would take up if it was all gas and no solid iodine. Any given mole of gas has a volume of 22.4 liters at standard temperature and pressure. Standard temperature and pressure is 0oC and 1 atmosphere. This is 32oF so this is a good assumption for the size of a Gastly in Minnesota this Halloween.
(0.35 moles) x (22.4 liters/mole) = 7.8 liters
If you wanted to this calculation at other temperatures, you could use the ideal gas law (which was featured in this blog post about “Deflate Gate” back in 2015. )
So, if you really had to catch ‘em all and get a Gastly, you could fit your new spooky friend into a 3 gallon (11 liter) jug – like the kind used for water storage or office water coolers – with plenty of room to spare.
If you play Pokémon Go, you can use the math presented here to figure out how many moles are in your Gastly (or Gastly’s evolution, Haunter). You can even use the periodic table to see how this would change if you assume Gastly is made out of some different gas! After all, a shiny Gastly is more blue than purple so perhaps those are made of some other gas.
Happy Halloween!
REFERENCES
- ThermoFisher Scientific. Materials Safety Data Sheet – Iodine. Revised May 2017.

[…] Sustainable nano: How many moles of gas are in a Pokémon Gastly?. https://sustainable-nano.com/2017/10/30/moles-of-gas-in-pokemon-gastly/ (accessed 2nd November […]
According to the pokédex entry in Diamond, “This Pokémon’s body is 95% made up of gases, which are blown away by strong gusts of wind.”
of course it doesn’t specify whether this is 95% by mass, this is by far the easier case.
95% of 90g is 85.5g of gas, x 1.254mol/g = 0.34 moles of gas.
0.34 mol x 22.4l/mol = 7.54 litres of gas
Alternatively, we could have calculated all of this by volume, but we get a wildly different answer. My Gastly’s height is listed as 1.3m, which clearly cannot be the height of the 5% solid bit, so I will guess it represents the diameter of a sphere containing the solid and gaseous parts.
1.3m diameter/2 = 0.65m radius. 4/3π0.65m^3 = 1.15 m^3, *1000 l/m^3 = 1150 litres*95% = 1092 litres of gas
1150 l x 1/22.4l/mol = 48.8 moles of gas
I don’t think it’s safe to assume Gastly is an ideal gas. It’s held together, so there must be attractive forces at play. Perhaps Gastly would be better fit to the Van der Waals equation of state?