Have you ever made lightning in a pickle? I have. It involves putting two iron nails in a pickle and applying 110 volts of electricity. It’s pretty smelly, but it is worth it to see what looks like yellow-orange lightning in the pickle.
What’s going on? The principle of “put in electricity – get out light” is fundamental to many display and lighting technologies, like the quantum dot materials we work with in the Center for Sustainable Nanotechnology. But how does it work? Let’s use the pickle to figure this principle out.
First, we need to say a few things about light. Usually we think of light as a wave, with a certain frequency and wavelength:
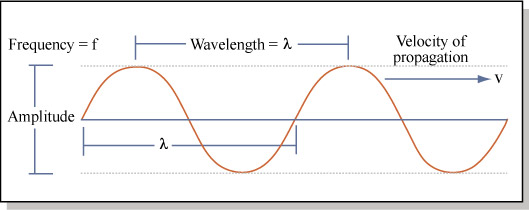
We have known for over 100 years the relationship between these parameters for light:
c = λν
Where c = the speed of light (3.00 x 108 m/s in a vacuum), λ = the wavelength in meters, and ν = the frequency per second (measured in Hertz).
But we can also think of light as a particle, a little packet of energy. We call this packet a “photon,” as in “Fire the photon torpedoes, Mr. Sulu!”

We also know how to relate the energy of a photon to its wavelike properties:
E = hν
Where E is the energy of the photon (in Joules), h is a constant (Planck’s constant, 6.626 x 10-34 J s) and ν is frequency again (in units of “per second” or Hertz, just like we saw in the wave equation).
If you think about it, it is pretty amazing that we can think about light as a particle and as a wave at the same time. That connection is a major concept in physics and chemistry.
But how does all of this relate to the pickle? Well, we can see that orange light comes out of the pickle when we put energy into it in the form of electricity. If we measured the wavelength of this light using a simple spectrometer, we would find that it is 589 nm, or 589 x 10-9 m. Knowing the wavelength, we can then calculate the energy of these “orange photons”:
E = hν and c = λν
so
E = h(c/λ)
E = 6.626 x 10-34 J s (3.00 x 108 m/s ÷ 589 x 10-9 m)
E = 3.37 x 10-19 joules

You might remember that a pickle started life as a cucumber and was then soaked in table salt (sodium chloride) solutions for a long time as part of the pickling process. One clue as to why the light is orange here: you can experiment with soaking a pickle in other salts, like potassium chloride or strontium chloride, and when you put 110 V through the pickle, you will get other specific colors of light! Therefore, the orange light is particular for sodium.
How can we understand this? Similar experiments were done over 100 years ago in vapor with many different elements, including sodium; when voltage is applied to two electrodes at the ends of a tube containing the vapor, sodium also produces 589 nm light.

Here is what we, the scientific community, eventually figured out:
- Atoms contain electrons that surround a nucleus. The electrons are particles that bear a negative charge and have a property called “spin.” (We represent the spin by writing an up-arrow or a down-arrow for the electron.)
- Electrons in an atom do not just have any random energy; they exist in states that have well-defined energies.
- We call these states “orbitals”, and they have certain labels like 1s or 2p (as shown in the figure below item 5). In addition to having a well-defined energy, each orbital has a certain 3D shape that corresponds to a location where we might actually find the electron. We won’t worry about the shapes of the orbitals now, though; we just need to know about the energies.
- When we put energy into an atom, we promote electrons from their lowest-energy state (the “ground state”) to an “excited state”:
-

Electrons in an atom can increase in energy from the ground state to an excited state (image by Rozzychan) Electrons fall back down to the ground state by releasing energy in the form of a photon of very specific energy: 3.37 x 10-19 joules in the case of sodium:
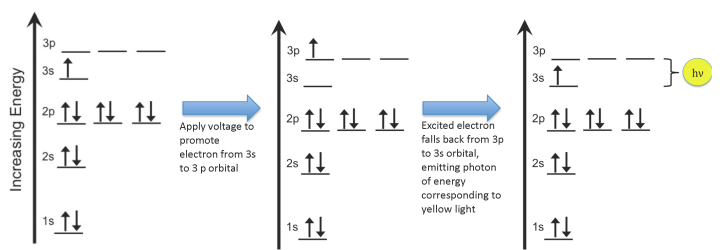
These ideas – that electrons have specific energies, you can promote them into higher-energy states, and then they relax back down to their ground state – are important for many applications that involve electrons, which basically means all of electronics, computers, sensors, screens, cell phones, etc. If electrons in an atom did NOT have specific energies, then we would see a whole rainbow of colors coming out of our electrocuted pickle. But we don’t: we see orange.
And since I am at the University of Illinois, whose school colors are orange and blue, I am still in search of the best way to get a pickle that emits both orange AND blue when I zap it with a voltage.

EDUCATIONAL RESOURCES
- Jefferson Lab Science Education: It’s Elemental- Element Math Game!
- James Madison University: Electrocute a Pickle classroom demo lesson plan
REFERENCES
Appling, J. R.; Yonke, F. J.; Edgington, R. A.; Jacobs, S.; Jones, R. F. Sodium D Line Emission from Pickles.Journal of Chemical Education, 1993, 70, 250-251. doi: 10.1021/ed070p250
Rizzo, M. M.; Halmi, T. A.; Jircitano, A. J.; Kociolek, M. G.; Magraw, J. A.; Dozark, D. Revisiting the Electric Pickle Demonstration. Journal of Chemical Education, 2005, 82, 545-546. doi: 10.1021/ed082p545
