The phrase, “only 90’s kids will remember” is thrown around a lot these days but it is safe to say most people born in the 80’s and onward have seen those big pits filled with colorful plastic balls. A staple of almost anyone’s childhood, this pit is, of course, the aptly named Ball Pit (Figure 1). As you jump around in this sea of balls, the force of your body sends these colorful plastic spheres flying everywhere.

However, while your initial jump into the pit may send balls from the top of the pit flying into the air, your body will never displace the balls deep below the surface of the pit (unless you actually “swam” to the bottom). The balls deeper down, while being pushed aside by the impact of your body, will just collide with the other balls closer to the surface but ultimately never escape to fly into the air.
This concept of only balls near the surface being able to escape the pit when they are impacted helps to explain why X-ray photoelectron spectroscopy is utilized for surface characterization. X-ray photoelectron spectroscopy, or XPS for short, is used to analyze the first 10 nm of a sample in order to determine what elements are on the surface of the sample (Hollander and Jolly, 1970). For perspective, a sheet of paper is about 100,000 nanometers thick! This type of spectroscopy occurs under ultra-high vacuum conditions. Ultra-high vacuum refers to a very low pressure (below 10-7 pascal). This is comparable to the pressure in outer space which has been estimated to range from between 0 and 10-11 pascal (source).
To analyze a sample, beams of X-rays are shot at a material, and these X-rays eject electrons from the atoms that make up the sample (Figure 2). These ejected electrons are referred to as photoelectrons, which are electrons that have been emitted from a sample due to that sample’s absorbance of electromagnetic radiation (i.e. light). We can measure this phenomenon thanks to an understanding of what is called “The Photoelectric Effect”, which is explained by the conservation of energy. The energy of the X-ray is used to provide the energy required to overcome the attraction between the electron and the nucleus of the atom (the electron’s binding energy); however not all of the X-ray’s energy is used in this process. The excess energy of the X-ray that is greater than the binding energy of the electron is now the energy with which the electron will leave the atom. Thus, energy is conserved! The equation used to characterize these electrons is EB=Ehv-Ek or binding energy is equal to the energy of the X-ray photon minus the measured kinetic energy of the electron. We can thank the photoelectric effect and conservation of energy for ensuring that all of the energy from the X-rays goes into moving the electron (kinetic energy is the energy of motion).
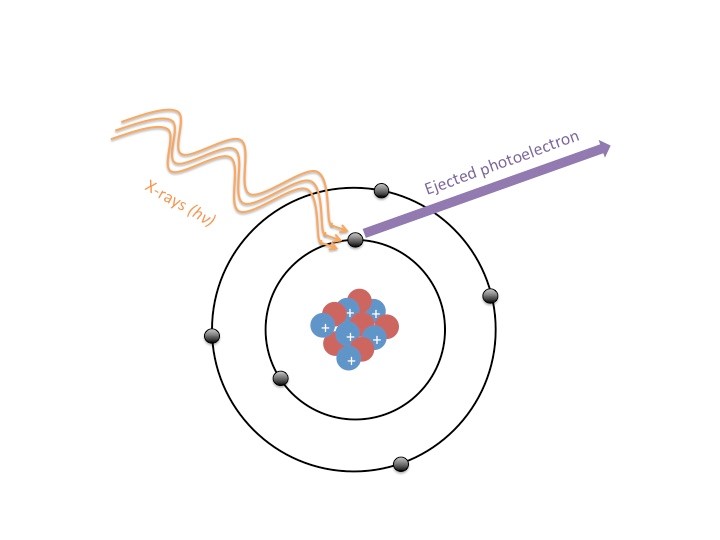
Although the X-rays reach deeper into the sample than 10 nm, the electrons captured by the detector are only the surface level electrons because any electrons deeper than 10 nm into the sample will collide with the electrons in the upper layers of the sample. So if we go back and imagine the ball pit, your body is like the waves of X-rays hitting the sample. Though you may sink further into the pit, you don’t send every single ball in the pit flying into the air, just the ones that you impact most directly.
A special device distinguishes the energy of the photoelectron ejected from the sample; this energy analyzer is a hemisphere (half-circle) consisting of two curved plates with different electrical potentials that result in the generation of an electrostatic field. Photoelectrons from the sample enter the hemispherical analyzer which ensures that only particles of a specific energy can reach the detector – meaning if we vary the energy of the analyzer, we count the number of photoelectrons emitted from the sample from a broad range of energies.

XPS is frequently performed in an ultra high vacuum environment due to the nature of photoelectrons. These small, highly energetic particles are constantly moving and doing so very quickly (hundreds of thousands of meters each second!). If they were to be ejected from the surface of a substrate in a room full of air, the photoelectrons would quickly collide with the nearest gas molecules (Figure 4). In an ultra-high vacuum, there is a negligible amount of gas molecules, and this ensures that the photoelectrons that reach the electron detector have not collided with (and lost energy to) gas molecules in the chamber. Going back to the ball pit analogy, having gas molecules in the chamber would be like having a series of disco balls hanging over your ball pit, so that when you jumped in, the small plastic spheres would bounce off of the disco balls and bounce back or ricochet. The disco balls act as a source of interference with the natural flight pattern of the displaced balls from the ball pit; just as gas molecules would interfere with the flight of the electrons.

So how exactly can the process described here be used to characterize a sample? Electrons from different elements will have characteristic energy levels since the binding energies of electrons differ from element to element. This difference in binding energy has to do with the structure of atoms. The probability of an electron being in a certain region of an atom is called an orbital; some electrons are in orbitals that are much further away from the nucleus than others. This depends on the size of the element, and how many protons and neutrons are in the nucleus (see Figure 2). Thus the strength with which these electrons are held (or binding energy) will be different for each element. So when a XPS scan is taken, the known energy of the X-ray photon and the kinetic energy of the electron detected by the electron acceptor will correspond to the unique binding energy for an element as demonstrated by the binding energy equation I laid out above.
XPS is a robust technique for determining what elements make up a sample, especially at its surface. This has many useful applications, including surface characterization, catalytic studies, and many more. One application that you probably use everyday is your cell phone! The reason that we were able to shrink the size of cell phones from the one in Figure 5 below is by using microelectronics, or very small wires, resistors, capacitors, anodes and more. XPS can be used to help characterize these microelectronics, allowing scientists to create better (and smaller!) cell phones. Understanding the electronic structure of something is key to understanding how we can manipulate it and how it will interact with other elements, atoms, and the surrounding environment. Using XPS to understand surface chemistry and binding environments can aid in scientific exploration that may just bring about innovative, and possibly smaller, technology.

RESOURCES
- http://www.npl.co.uk/science-technology/surface-and-nanoanalysis/surface-and-nanoanalysis-basics/introduction-to-xps-x-ray-photoelectron-spectroscopy
- http://xpssimplified.com/whatisxps.php
- Hollander & Jolly, 1970. X-Ray Photoelectron Spectroscopy. Chem. Res., 3:6, pp 193-200. http://pubs.acs.org/doi/pdf/10.1021/ar50030a003
