As Kermit the Frog says, “It’s not easy being green.” But for plants and exuberant celebrators of March 17, it is very natural. The color green becomes very prominent in the month of March as St. Patrick’s Day produces extravagant festivities coated in the color. The beginning of spring brings the blooming of flowers and growth of leaves. The onslaught of green becomes evident all around and especially in the forest. The component responsible for much this green color in plants – and some important life processes – is chlorophyll.
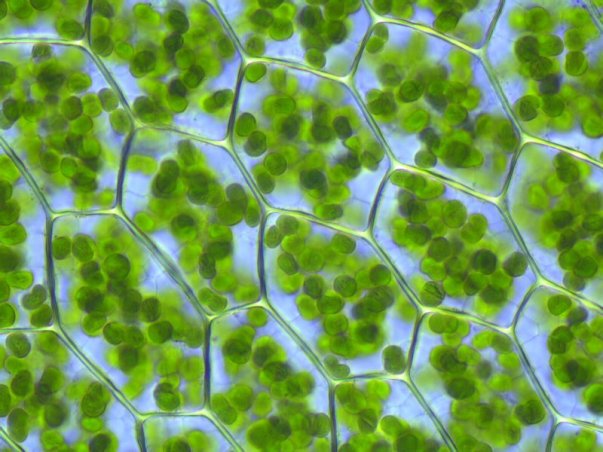
Chlorophyll comes from two Greek words: χλωρός (chloros) and φύλλον (phyllon), which mean “green” and “leaf,” respectively.1 These words are a good fit based on the properties and location of chlorophyll. Chlorophyll can actually be any of several green pigments that are found in the chloroplasts of plants. It is vital for the life of many plants because it is what absorbs light and turns it into energy (photosynthesis).

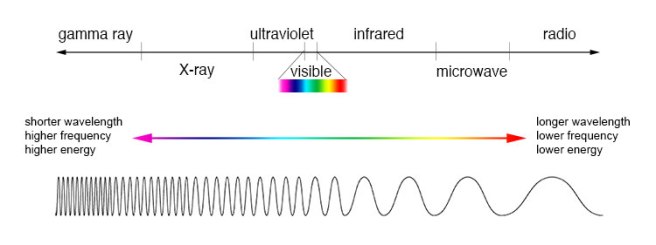
So how does chlorophyll give plants their green color and why do plants need it? To do the job of photosynthesis, chlorophyll absorbs visible light in the blue and red regions of the electromagnetic spectrum, but generally reflects wavelengths in the green region. This reflected green light is what makes its way to our eyes. (For more on how we see color, see this previous blog post.)
But giving off a nice green color is incidental to chlorophyll’s role in providing the necessary photosynthesis that most plants cannot do without. The chlorophyll uses the energy it absorbs from sunlight, along with carbon dioxide and water, to produce oxygen gas and sugars (energy!). They also can end up as structural components of the plants like cellulose. (A nanostructured version of cellulose called nanocellulose2 is important in some of our Center for Sustainable Nanotechnology research – you can learn more on that from our podcast episode Making Sustainable Nanoparticles from Plants.)
Chlorophyll is not the only source of color (pigment) in plants, but in many plants its green color overshadows the color of other pigments. In the autumn months, the days start to get shorter and therefore less chlorophyll is exhibited in the leaves of trees. This allows for these other pigments to show their true colors and trees start to show their beautiful reds, oranges, and yellows. It benefits plants to have multiple pigments because this allows them to absorb all the visible regions of light as opposed to just the blue and red regions that chlorophyll absorbs.

The light energy that is absorbed by chlorophyll doesn’t always go through photosynthesis. Some can also be re-emitted as light (though not always visible), and some can be emitted as heat energy. These three processes compete against each other: if there is an increase in one, then there will generally be a decrease in one or two of the others For example, if more light is absorbed for photosynthesis, then less will be re-emitted as light or heat.
Believe it or not, this balance between photosynthesis, light emission, and heat emission is related to nanotechnology research. The research technique of chlorophyll fluorescence looks at the light emitted from chlorophyll to learn more about photosynthesis and heat loss,3 and this technique has been used to determine the effect that silver nanoparticles have had on plants. For example, a team of researchers from Brazil and the UK found that silver nanoparticles suppressed the fluorescence of chlorophyll, which could mean that the chlorophyll was not doing as much photosynthesis. They also found that the fluorescence decreased even more as the nanoparticles got bigger.
One possible explanation for this would be that the chlorophyll molecules are being bound to the surface of the nanoparticles and transmitting their energy to the silver surface instead of converting it into sugar. This could mean that silver nanoparticles inhibit photosynthesis, if some of the energy that would normally go toward photosynthesis instead goes to heating the silver nanoparticles.4 The study shows that the chlorophyll fluorescence method can be used for studying of the effects of nanoparticles on plants, and may be helpful in building more sustainable nanoparticles in the future.

There is a lot to the greenness of plants that people don’t necessarily think about everyday. So this month when you are decked out in your festive green clothing, remember “going green” has multiple meanings, including protecting the natural greens of the plant kingdom.
EDUCATIONAL RESOURCES
- ACS activity: Coloring with Foods (grades 3-5)
- NASA lesson plan: Discovering Color with a Prism (grades 9-12)
- Science & Plants for Schools: Photosynthesis – A Survival Guide for Teachers (ages 11-14)
REFERENCES
- Chlorophyll. Wikipedia. https://en.wikipedia.org/wiki/Chlorophyll.
- Garret, R.H. and Grisham C.M. Biochemistry. Brooks/Cole. 2013.
- Maxwell, K and Johnson, G.N. Chlorophyll fluorescence—a practical guide Journal of Experimental Botany. 2000, 51 (345), 659-668. doi: 10.1093/jexbot/51.345.659
- Querioz, A.M. et al. Quenching of chlorophyll fluorescence induced by silver nanoparticles. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2016, 168, 73–77. doi: 10.1016/j.saa.2016.05.033
