‘Tis the season for snowy weather here in the Upper Midwest! There are many ways to enjoy snow, but ever since my parents strapped a pair on my feet at the mature age of 3 years old, my favorite way to celebrate winter has been on skis. From carving downhill (like Figure 1a) to cranking out cross-country ski marathons (like Figure 1b and d) — it is all great winter fun! I’m certainly not the only one to think that either: approximately 50 million people skied in the US during the 2015/2016 season1 and they spent about $4.6 billion dollars on gear to do it.2
And it gets even better: it turns out that skiing is not only a great winter activity, but it also intersects in many ways with the nanomaterial world. In this post, I’m going to talk about some of those intersections and how nanomaterials make winter wonderlands that much more fun!

First things first: to understand what nanotechnology has to do with skiing, we need to talk about how skis interact with snow on a molecular level. You can read more about snow in a previous blog post (The Science of Snow), so I’m going to focus on the skis for now. If you survey a range of modern skis you’ll find that, though there are different designs across different styles of skiing (see Figure 1 for examples!), they all share a common material on the surface of the ski that faces the snow: polyethylene.
Polyethylene (PE) is the most common type of plastic and it’s used for loads of stuff from bottles to storm drains to hip replacements. PE consists of carbon atoms linked together in a chain, where each of the carbon atoms has two hydrogen atoms bonded to it (Figure 2).

Different numbers of carbon atoms in the PE chain can give the molecule different properties. For example, extremely long chains—like 250,000+ carbon atoms long—create a tough material useful for products like skis. On the other hand, shorter chains make for soft plastics useful for things like bags. These changes in the PE’s strength as a function of chain length are due to attractive forces between chains called Van der Waals forces. Van der Waals forces are very weak between small molecules but may become enormous as the molecules get bigger. (For previous blog posts about Van der Waals forces, see here and here.)
All the fun (and sometimes the terror) in skiing comes from low friction between the skis and the snow. Polyethylene is hydrophobic—it’s scared of water! Liquid water will bead up and roll off of its surface and this is the driving factor in why ski bases can glide over snow. Well, hold on a second: you might be asking, how can snow, solid crystalline water, bead up and roll off of a ski??? That seems impossible.
The answer is that, in most conditions, you aren’t actually skiing on snow crystals but on a thin layer of liquid water that forms as the ski base glides over the snow surface. Ski bases heat up as they glide across snow and a very thin layer of water forms between them.3 You can feel this kind of frictional heating yourself by rubbing your palms together and feeling your hands warm up! When weather conditions are right (neither too cold and dry nor too warm and wet), the layer of water formed on the base of the ski lubricates the rest of the ski as it glides over the snow, there’s minimal friction, and everything is dandy. This “just right” situation of a thin layer of water on the ski base is shown in the center third of Figure 3 below.4
Conditions can be outside that “just right” range in a variety of ways. A layer of water thicker than the friction layer can be created by environmental factors like bright sunlight, warm air temperatures, and high humidity. Unfortunately, this can be too much of a good thing. If you’ve ever gone spring skiing you may have noticed that your skis are sort of ‘suctioned’ to the snow. This is called capillary drag and, for example, it is the same process that is allows paper towels to soak up spills.
As we learned in The Science of Snow, water molecules are capable of hydrogen bonding (a ‘molecular stickiness’ that allows water molecules to hold on to one another). Capillary drag occurs when these water molecules stick to not just each other but also to the ski base. Suddenly you aren’t just skiing over a water layer but you are dragging it along with you (right third of Figure 3). That’s hard work!
Finally, if you’ve ever tried to ski in very cold conditions, you may have noticed that your skis don’t glide very fast. This is because it is much harder to melt a lubricating water layer at very low temperatures. As a result, there’s a lot of dry friction from the skis plowing over sharp snow crystals (left third of Figure 3). That’s also hard work!

As you can imagine, the multibillion-dollar ski industry has put a lot of thought into how to reduce friction between skis and snow so that we can enjoy skiing all winter long. Most of their focus has been on reducing capillary drag by using waxes on the ski base to increase the hydrophobicity of the polyethylene.5 The majority of these waxes can be placed into three categories:
- Hydrocarbons: chains of carbon atoms bonded with hydrogen atoms. These are much shorter than the ones that make up the PE ski base. (Figure 4a)
- Fluorocarbons: chains of carbon atoms bonded to fluorine atoms. An example fluorocarbon you’ve probably heard of is Teflon. (Figure 4b)
- A combination of hydrocarbons and fluorocarbons known as diblock copolymers: Don’t be intimidated by this jargon! “Copolymer” refers to a molecule that is made from bonding two or more unique molecules together in a repeating fashion, and “diblock” just means that there are only two unique units in this particular copolymer. (Figure 4c)

There’s more than a little pseudoscience/magic/superstition involved in choosing waxes for a given set of snow conditions (let alone how to apply them to skis). There is not a lot of research about what exactly the waxes do on the molecular level once they are on the skis. But the general motivation behind the process is to clean any friction-causing debris off the ski base and to impregnate the PE with the chosen wax so that the hydrophobicity of the base is maximized in wet conditions.

In a laboratory, scientists study the hydrophobicity of a material by measuring the contact angle of a droplet of water on the surface. The contact angle is the angle between a water droplet’s outer edge and the surface it’s sitting on (Figure 6). A surface is considered hydrophobic if this angle is greater than 90o and hydrophilic (water loving, liquid water will wet the surface) if it is less than 90o. You can see in the figure below how the hydrophobic droplet looks more “beaded up” on the surface, while the hydrophilic droplet is more spread out.

Hydrocarbons, including the PE ski base itself, have contact angles just over 90o, which just barely qualifies them as hydrophobic. Fluorocarbons like Teflon6 have contact angles around 108o, which is an improvement… but nature came up with something much better —superhydrophobicity—and it works on the nanoscale!
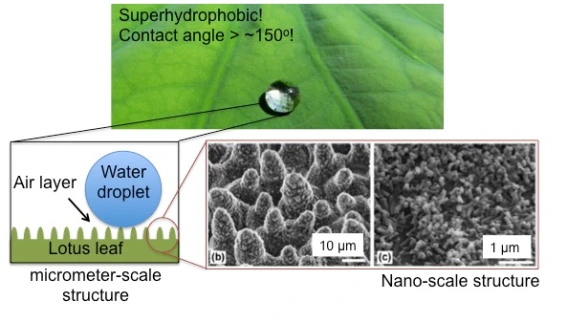
A material is considered superhydrophobic when the contact angle is greater than 150o. You can check out one of our previous blog posts that mentions the archetype of superhydrophobic materials, lotus leaves, here. In summary, the micro- and nano-structures on the surface of a lotus leaf create an air layer between water droplets and the leaf’s surface (Figure 7). This makes for a very high contact angle and a very hydrophobic surface.
The ski wax industry has taken a leaf from the lotus book (hehe) and started manufacturing ski waxes inspired by the structures of lotus leaves. Holmenkol, a German ski wax company, advertises a patented “Lotus-Hybrid-Matrix” technology that combines nanomaterials with ski waxes to achieve lotus-like water repellency on ski base surfaces. They are light on details in their website (understandably, due to proprietary trade secrets), but Holmenkol does describe their “Nano-CFC” technology as a fluorocarbon wax with nanocomposite additives. Some other companies that use nanotechnologies include:
- Swix, a Norwegian company known for being the first science-minded producer of modern ski waxes (as early as 1946),8 has also begun advertising the incorporation of unspecified nanotechnology into waxes for dry friction conditions.
- Toko, a popular ski wax brand in central Europe that was acquired by Swix in 2010,9 used to manufacture a line called “Nano Tec” that was a spray-on liquid wax with presumably some nano-inspired component.
- Star, a company based in Italy, has been leveraging nano-scale ceramics,10 like tungsten disulfide, under the marketing name of “Cera-Flon.” It’s hard to tell what “Cera-Flon” is, chemically speaking, but a quick Google search leads me to believe it is some combination of a fluorocarbon polymer and ceramic that has been used for other non-stick surfaces like cookware.
- Start (Finland) also incorporates “nano-fluoro particles” into some of their waxes and touts their self-cleaning abilities.11
- Peltonen, also a company out of Finland, sells a ski with a “Nanogrip” coating that they claim enjoys all the benefits of nanotechnology without additional waxing!
As you can see, there are quite a few nano-influenced ski products on the market today. I’ll admit that nanomaterials aren’t the first thing I think about when I’m playing around in the snow, but by the end of a 50 kilometer ski marathon I certainly am appreciative of all the research that goes into making skiing faster, more efficient, and more fun!
Happy winter everyone!

EDUCATIONAL RESOURCES
- Teach Engineering: Superhydrophobicity — The Lotus Effect (grades 10-12)
- Science Buddies: Skiing and Friction: How Does Ski Wax Affect the Sliding Friction of Skis?
REFERENCES & FOOTNOTES
- Report by National Ski Areas Association: http://www.nsaa.org/press/pressreleases/skier-visits-up-to-539-million-in-2015-16/
- Report by Snow Industries America: http://www.snowsports.org/research-surveys/snow-sports-fact-sheet/
- Colbeck, S.C., Bottom Temperatures of Skating Skis on Snow. Journal of the American College of Sports Medicine, 1993, 258-262. PMID: 8164546
- Colbeck, S.C., A Review of the Friction of Snow Skis. Journal Sports Sciences, 1994, 12: 285-295. doi: 10.1080/02640419408732174
- It’s significant to mention that the ski wax industry has also put significant effort into putting millimeter-scale patterns into the polyethylene bases to reduce capillary drag by giving the water layer between the ski and the snow less surface area to stick to. That’s another blog post’s-worth of information for a different day 🙂
- Maybe this thought came to mind while you were reading: “If hydrophobicity is so important, why don’t they make ski bases out of Teflon? Why mess around with polyethylene at all?” I wondered this for a long time too, until I got my hands on some Teflon. It’s a surprisingly soft plastic that can be indented and scratched with a fingernail—not so good for a ski base that need to withstand repeated runs over ice and trail debris like sticks and leaves. Also, and perhaps even more importantly, Teflon is pretty darn expensive ($208.65 for 144 cubic inches) compared to polyethylene ($37 for 144 cubic inches) according to eplastics.com.
- Ensikat, H.J., Ditsche-Kuru, P., Neinhuis, C., Barthlott, W. Superhydrophobicity in perfection: The Outstanding Properties of the Lotus Leaf, Beilstein J. Nanotechnol. 2011, 2, 152–161. doi: 10.3762/bjnano.2.19
- Masia, S. Grip and Glide: A Short History of Ski Wax: https://www.skiinghistory.org/history/grip-and-glide-short-history-ski-wax
- Herz, N. After Acquisition, Changes at Swix and Toko Will Be Behind the Scenes: http://fasterskier.com/fsarticle/after-acquisition-changes-to-swix-and-toko-will-be-behind-the-scenes/
- Ceramics are pretty neat because they can act as a dry lubricant. Tungsten disulfide has a layered structure where the bonding between the layers is weak. This allows the layers to slide past one another easily, thus acting like a lubricant. Check out more on Wikipedia.
- Waxing Guide for XC Skiing: http://www.startskiwax.com/application/files/8814/5639/0970/start_waxing_guide_for_xcountry_skiing_en.pdf
