By now you’ve probably heard about Samsung’s recall of all Galaxy Notes 7s. Several years ago the entire worldwide fleet of 787 “Dreamliners” was grounded due to onboard battery fires. You might be wondering, “Why are all these batteries catching on fire? Are lithium ion batteries safe?”

To answer this, let’s look at how a lithium ion battery is made. The battery contains four main parts: two electrodes (positive cathode and negative anode), the electrolyte, and a separator.

The positive electrode is made from a metal oxide, usually LiCoO2 (lithium cobalt oxide), and the negative electrode is made from graphite. The basic idea in the battery is that both LiCoO2 and graphite are compounds that have a sheet-like structure, which allows lithium ions to slip in between the sheets. To keep air and moisture out, the electrodes are sealed inside a plastic bag, forming a “pouch cell.”

The figure below shows the situation when you are recharging the battery: When you are charging the battery, an external voltage from your charger pushes the Li ions out of the LiCoO2 cathode; they pass through the electrolyte, and slip in between the sheets of the graphite anode. Later on when you use the battery, you are slowly letting the lithium ions come back from the graphite to the LiCoO2, and for each Li ion that comes back, one electron gets pushed through the external circuit to power your laptop or cell phone. (For even more about how Li-ion batteries work, check out this blog post.)
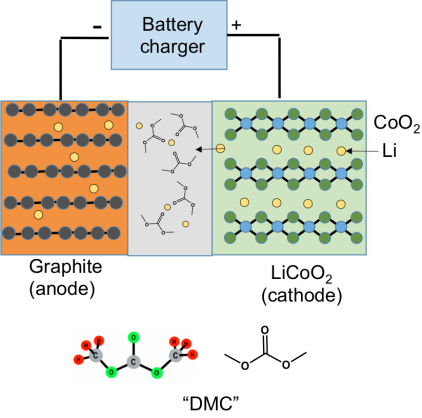
The separator between the electrodes is needed because there’s a voltage difference between the anode and cathode. If they touch, the battery will have a “short circuit” and malfunction (more on that below). Finally, the “electrolyte” is an organic liquid that contains an ionic compound called a salt. The organic liquid is made from a mixture of compounds that are chemical derivatives of CO2. The diagram above shows one of these molecules in the gray separator area, dimethyl carbonate (DMC). DMC and related alkyl carbonates are needed to shuttle the Li ions between the electrodes, but DMC is also highly flammable. (Important disclosure: in 2007 I co-founded a company, Silatronix, that is making new, non-carbonate-based electrolytes to improve battery safety and performance, and so I have a financial as well as an academic interest in battery safety.)
Now that you know how a battery is supposed to work, let’s get back to the question at hand: Why are Samsung batteries catching on fire? There are a number of possible answers, but here are the three most important ones:
1. Separator failure
As mentioned earlier, if something causes the anode and cathode to touch, then there will be a “short circuit”. The battery will be rapidly discharged through the very small contact area. That area will get very hot, causing the electrolyte to break down and catch on fire. Separator failure can be caused by mechanical damage and can also be caused if you leave the battery charging all the time, which can cause lithium to form little needle-like “dendrites” that can poke right through the separator.
2. Cathode degradation
The cathode in most batteries is made from LiCoO2. There is a push to replace this with other materials, notably mixed oxides like LiNiMnCo (“NMC”) or LiNiCoAl (“NCA”), but for now the batteries in most of our everyday electronics like phones and laptops will almost certainly contain LiCoO2. When a battery is over-charged (charged at a higher voltage than it was designed for), too many lithium ions are pushed out of the LiCoO2. Under these conditions, the LiCoO2 can release oxygen (O2) and transform to a new compound, Co3O4. While mixing of O2 with the highly flammable Co3O4 mixture is bad all by itself, it’s also notable that Co3O4 is worse at conducting electricity than the original LiCoO2. This means that when the battery is discharged and/or recharged the next time, there’s more heat within the battery because the resistance of the cathode acts like a little heating element inside the battery. If you continue to try to push the Li ions out, it will get hot enough to start a fire. Overcharge can happen if you use the wrong battery charger or if you inadvertently swap batteries that are designed for different operating voltages.
3. Electrolyte breakdown and cell pressurization
The electrolytes are made from molecules that are derivatives of carbon dioxide. When the battery is charged, there’s some possibility of breaking down these molecules, releasing CO2. While CO2 is itself pretty innocuous, remember that a lithium ion battery is sealed up tight, so the CO2 pressurizes the battery from the inside, like a can of soda left in a hot car. If something causes the battery to burst, there can be a sudden release of the gas and the highly flammable electrolyte.
So, are lithium ion batteries safe? In general use, yes. However, the presence of flammable compound inside always leads to the possibility of a fire. It’s important to treat your batteries well. For example, always make sure that you charge them to the proper voltage, and don’t swap different chargers. It’s best not to leave them on the charger any longer than necessary, as this also decreases battery lifetime. Don’t leave your cell phone in the hot sun, since high temperatures usually accelerate the rates of chemical reactions, including those that lead to battery failure and/or battery fires. If your battery is getting hot, then something’s wrong and you should have it replaced. Finally, please make sure you never put your lithium ion batteries in your checked baggage on an airplane!
I don’t work at Samsung so I can’t say exactly why the Galaxy Note 7 is particularly prone to combustion. We’ll probably have to wait a while before the final verdict is public, but it seems likely that there is a flaw in manufacturing or design related to one or more of the three types of failure I listed above. In the case of the famous 787 Dreamliner fires, the National Transportation Safety Board issued an analysis that is public information, but it was inconclusive. However, that report has many photographs showing the internal construction of larger-format batteries.
The bottom line: Enjoy your mobile electronics, but remember that anything that stores energy – whether it’s a battery in your cell phone or a tank of gas in your car – has the potential for the energy to be released in an unanticipated way. So be good to your batteries!
Robert Hamers is Director of the Center for Sustainable Nanotechnology. He is also co-founder and Chief Science Officer of the start-up company Silatronix.
EDUCATIONAL RESOURCES
- Rechargeable Battery Recycling Corporation Battery Lesson Plan (grades 5+)
- Earth Day Canada: Battery Basics lesson plan (elementary)
ADDITIONAL RESOURCES
- Battery University: very good information about battery science and technology – http://batteryuniversity.com
- Scientific American: article about how batteries grounded the Boeing 787 Dreamliners – https://www.scientificamerican.com/article/how-lithium-ion-batteries-grounded-the-dreamliner/
- National Transportation Safety Board: analysis of 787 Dreamliner battery fires – http://www.ntsb.gov/investigations/AccidentReports/Reports/AIR1401.pdf
- Science Direct: Open-source technical article about the mechanisms of battery failure related to the cathodes – http://www.sciencedirect.com/science/article/pii/S0921510714002657

[…] Click the link below to learn more about the science behind these exploding batteries and what you can do to prevent a tragedy. https://sustainable-nano.com/2016/10/13/flaming-cell-phones/ […]
Bob: Thanks for the information. Would like to “Press” this to my site.
Great article Bob, Larry is very interested in this, he does fire investigations! Thanks, Sue
Sounds good -glad you found it useful !
Cool, Bob! Could see a second part on why my phone battery dies when I have it in pocket skating in the subzero Minnesota winter. Always wondered about that.
I agree! Inquiring minds want to know…