Along with eight colleagues (most from the Center for Sustainable Nanotechnology) I recently co-authored an article in the American Chemical Society’s Chemistry of Materials journal titled “Impact of Nanoscale Lithium Nickel Manganese Cobalt Oxide (NMC) on the Bacterium Shewanella oneidensis MR‐1.”1 Just as the name suggests, we analyzed how nanoscale NMC, an important material in some lithium-ion batteries, affected the growth and survival of an important soil dwelling bacterium. Since its publication, it has received some media attention for being the first scientific study characterizing the direct influence of battery materials on an organism – you can read more about the press coverage here and here.
As great as a TV interview is for getting the word out about our project, there was a lot more to the study than we had time to explain in those segments. The goal of this blog post is to answer a few more detailed questions about the paper.
Why study nanoscale lithium nickel manganese cobalt oxide (NMC)?
First of all, NMC is part of a class of battery cathode materials that provide high performance and power, making it well suited for use in electric vehicle batteries. For example, the 2015 Nissan Leaf’s 30 kWh battery, which replaces the older model’s 24 kWh battery, was made possible by replacing the old lithium manganese oxide (LMO)-based battery with an NMC-based battery.2 We know from a press release that LG Chem, which makes batteries for both the Nissan Leaf and Chevrolet Volt, is licensing the technology for NMC from 3M. Even more importantly for our research, materials like NMC are increasingly being implemented in nanoparticle form to improve battery performance and are on the cusp of large-scale commercialization.

We wanted to look at the biological impact of nanoscale NMC because not only will future generations of commercial NMC likely use nanoparticles, but currently manufactured NMC used in electric vehicle batteries already produces small nanoparticles when it undergoes stress-induced fracturing during use.
Why look at such a specific type of bacteria?
We chose to study a bacterium known as Shewanella oneidensis MR-1, a gram-negative bacterium that lives in sediment and soil. (For more on gram-positive and gram-negative bacteria, see this blog post.) The genus Shewanella is distributed globally and it plays a role in cycling metals in the environment. Bacteria are essential to the functioning of healthy ecosystems and may be some of the first organisms to encounter a material such as NMC in a landfill or disposal site. That means that testing how bacteria respond to NMC nanoparticles in the lab can give us insights about what might happen out in the real world.
How do you analyze the biological impact of NMC?
First we made nanosheets of NMC from scratch so we could control their size and composition. Then we examined their interaction with Shewanella oneidensis bacteria using two techniques: respirometry and optical density measurements. These two techniques compliment each other by providing slightly different information about how the bacteria grow and reproduce.
Respirometry allowed us to see how much oxygen was consumed by the bacterial culture as it grew. Healthy bacteria will reproduce and very quickly form an even larger population: their growth is exponential! As the population grows, the bacteria use up more oxygen. We can directly measure how much oxygen is taken up by the bacterial cells and correlate that to the bacterial population size. We noticed that bacterial cells exposed to NMC at a concentration of 5 mg/L initially took up less oxygen and did not grow as quickly as bacterial cells that were not exposed to NMC. When we bumped the NMC concentration up to 50 mg/L, the bacteria didn’t consume any oxygen at all — they were dead.
Optical density measurements allowed us to measure how turbid the bacterial culture was. More turbidity means there are more bacteria scattering incoming light. The less turbid and clearer the solution, the less bacteria were there to scatter light. We saw that when Shewanella oneidensis MR-1 was exposed to NMC, they scattered less light than when there were no NMC. This was another confirmation that NMC inhibited the growth of bacteria.

How does NMC impact the growth and survival of Shewanella oneidensis?
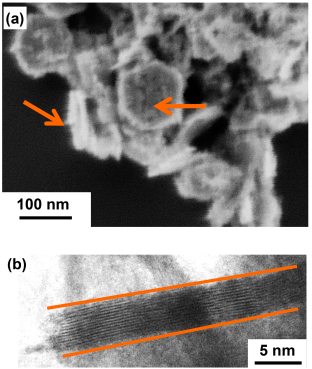
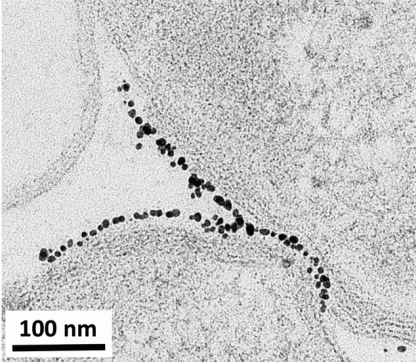
Initially, we thought that nanosheets of NMC might actually slice into the bacterial membrane, kind of like a paper-cut! To test this theory, we looked at NMC and the bacterial cells under a high-resolution electron microscope. However, we saw that the cells and nanosheets were not stuck together, making our paper-cut theory unlikely.
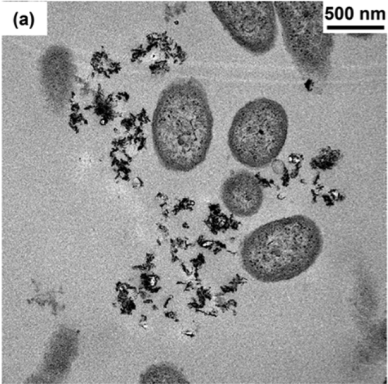
To figure out what other aspect of the NMC might be responsible for inhibiting bacterial growth, we then measured the composition of the solution that NMC was placed into. In other words, we tested the liquid where both the NMC and the bacteria were floating, and we saw that over time the NMC partially dissolved, releasing its component species such as nickel and cobalt into the solution. We measured the amount of metals released from NMC and then did an experiment where we exposed bacterial cells to only these metals by themselves, without any NMC. We discovered that the cobalt and nickel released from NMC were the main culprits in affecting bacterial growth!
What did we hope to achieve with our paper?
Our study provides new insights into how NMC and related lithium-ion battery cathode materials might interact with organisms in the environment. Ultimately, our goal is to use the results of our study to help design new NMC materials that are more environmentally benign and can be used sustainably in important applications such as energy storage in batteries.
Our study also suggests that consumers and companies should consider recycling NMC materials to prevent them from ending up in landfills and leaching metals into the environment. There is currently little economic incentive and no federally mandated system for recycling such materials, but (as we’ve discussed in an earlier post) as we use more and more of these materials in consumer products, it will become increasingly important to have infrastructure available to recycle them.

This post is part of our ongoing series of public-friendly summaries describing research articles that have been published by members of the Center for Sustainable Nanotechnology. This collaborative study was done by researchers from the University of Wisconsin-Madison and the University of Minnesota-Twin Cities. Mimi Hang and Ian Gunsolus, doctoral students at UW-Madison and the U of MN, were the paper’s co-first authors. (Ian has since graduated!) The article was first published online in January 2016 in Chemistry of Materials.1
EDUCATIONAL RESOURCES
- Hang, M. Gunsolus, I., Wayland, H., Melby, E., Mensch, A., Hurley, K., Pedersen, J., Haynes, C., & Hamers, R. Impact of Nanoscale Lithium Nickel Manganese Cobalt Oxide (NMC) on the Bacterium Shewanella oneidensis MR-1. Chemistry of Materials. 2016, 28, 1092-1100. doi: 10.1021/acs.chemmater.5b04505
- Frost, L. “Exclusive – Nissan faces battery plant cuts as electric car hopes fade.” Reuters Sep 15, 2014. Retrieved from http://uk.reuters.com/article/uk-renault-sa-nissan-batteries-idUKKBN0HA0CC20140915
- Feng, V.Z.*, Gunsolus, I.L., Qiu, T.A., Hurley, K.R., Nyberg, L.H., Frew, H. Johnson, K.P., Vartanian, A.M., Jacob, L.M., Lohse, S.E., Torelli, M.D., Hamers, R.J., Murphy, C.J. & Haynes, C.L.* Impacts of gold nanoparticle charge and ligand type on surface binding and toxicity to Gram-negative and Gram-positive bacteria, Chemical Science, 2015, 6, 5186-5196. doi: 10.1039/C5SC00792E

[…] original en inglés por Mimi Hang Traducido por Jeremy […]