My typical day (maybe like yours) involves waking up, taking a 10 minute shower, cooking breakfast, running the dishwasher if it’s full, going to work, eating dinner with a refreshing glass of filtered water, and maybe tackling a load of laundry in the evening. None of these actions feel extravagant, but when I look at statistics of global water usage and the lack of fresh water availability, it’s obvious that as Americans, we consume significantly more gallons of water per day than anywhere else in the world. In fact, on average each American uses about 152 gallons of water daily, while people in some other countries such as Uganda and Haiti use only about 4 gallons.1
That extreme low usage in some countries is not just because people are very conservation-minded – it is largely because there is not enough clean water to go around. Living in Wisconsin, I am conscientious of my water intake, but I am fortunate to not be in constant fear of turning on the faucet to see no water or even dirty water pouring out. Unfortunately, as we’ve learned from the recent experience of people in Flint, Michigan, it is not only countries outside the U.S. that have to worry about availability of clean water.
Personally, a trip to Israel last winter was what forced me to step out of my typical routine and experience firsthand how precious water is to their nation as a natural resource. My guide on the trip encouraged us to shower efficiently, never leave the water running while doing dishes, and to purchase bottled water, but to never waste a drop.

Being a scientist and an advocate for reducing my footprint on the environment, I wanted to learn more about what is being done to solve the problem of global freshwater availability. Considering what other recent medical and energy-related advancements have been made with the use of nanotechnology, it came as no surprise to me that carbon nanotubes (CNTs) are being studied as a means of water purification.
What exactly is a carbon nanotube? Picture a flat sheet of carbon atoms rolled into an incredibly tiny cylindrical tube with a diameter on the nanoscale, as shown here:
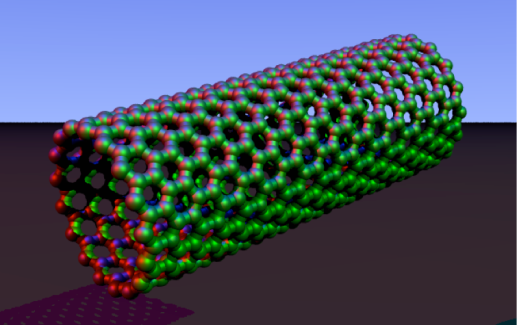
These tiny, flexible, and surprisingly resilient materials may very well be our new direction in sustainable, large-scale water purification. The major advantage of CNTs is that water passes through them in a nearly frictionless manner due to something called hydrophobicity (i.e. preference to be away from water). But if CNTs are hydrophobic, you might wonder why water would even come near or enter them in the first place. This is an important point because water is a polar molecule and CNTs are non-polar, meaning they typically don’t want to mix together. One option for overcoming this is to coat the top of each tube with specific molecules that will initially attract water to the opening. Then, when water enters the nanotube, it flows through very quickly because it is being repelled by the hydrophobic tube walls. Conversely, most salts, ions, and pollutants won’t flow through the nanotube because they are attracted to and captured by the coating at the opening.
Because water passes through the CNTs so easily and pollutants do not, CNTs have potential to be a great solution for water purification. If we’re able to use CNTs in the next generation of water purification technology, then we may be able to both desalinate (get rid of salt) and remove pollutants from otherwise unusable water around the world with less energy and less money than current methods require.
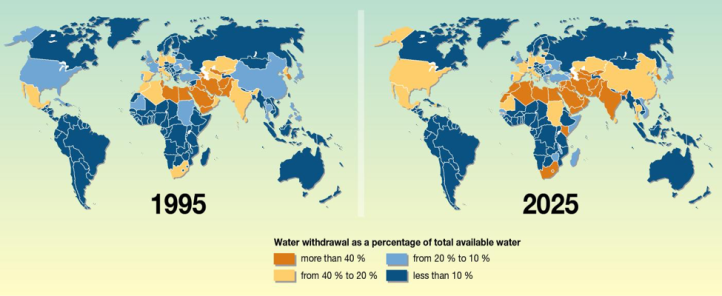
Improving water purification really is a global issue. On my trip last winter, I learned that Israel, along with other nations in the Middle East and Northern Africa, were already considered “water stressed” in 1995. This phrase indicates that the nation is withdrawing more than 25% of their renewable freshwater resources for agricultural, domestic, and industrial uses annually. By the year 2025 it is predicted that over 2.8 billion people in 48 countries will be “water stressed” or worse.2 To combat this growing issue, countries are turning to unconventional options for wastewater treatment and desalination.
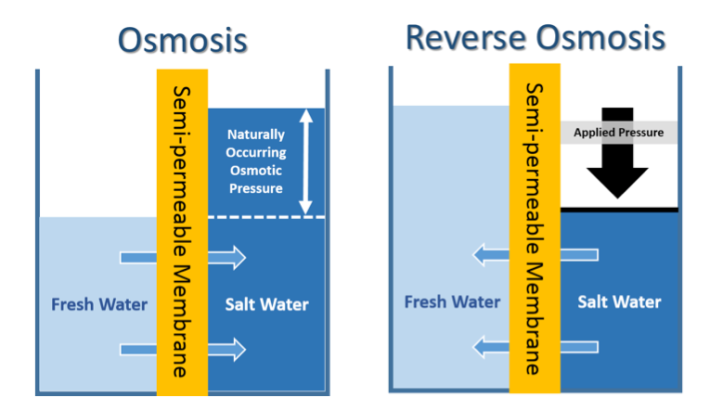
With a greater understanding of the problem at hand, let’s take a closer look at current techniques for water purification. Right now, the most common practice of cleansing salt water to produce freshwater is the use of desalination plants. These plants operate by the technique of reverse osmosis, which is shown in the figure below. Osmosis is when a less concentrated liquid (like fresh water) moves through a membrane toward a more concentrated liquid (water containing salt in this case). This process happens naturally without any need for applying external pressure. For reverse osmosis, we want the exact opposite to happen, and unlike osmosis, that requires an input of external energy. These semi-permeable reverse osmosis membranes that separate the two liquids are usually made of common organic materials with pores ranging from 0.3 to 0.6 nanometers in diameter. (As a comparison the diameter of a dime is 18,008,600 nanometers while the diameter of a water molecule is a mere 0.1 nm).
As of 2013, there were over 17,000 plants worldwide providing more than 300 million people with desalinated water, many using reverse osmosis technology.3 Israel alone, with its relatively small land mass, has four plants with a fifth currently being built, while the U.S. houses about 250 plants.4
Desalination plants are effective, yet they are costly monetarily, energetically, and environmentally speaking. These plants require a great deal of energy input daily, and most desalination plants run on non-renewable energy sources (like fossil fuels and nuclear energy).5 With global freshwater availability declining, there is a need for cheaper, more efficient, and more environmentally sustainable desalination technology, and CNTs may be our most viable option looking forward. Their desalination capacity and frictionless water interaction means they require less energy than reverse osmosis to produce the same amount of fresh water.

There are a few hurdles that must be overcome before we hear about the next “CNT desalination plant,” though. For example, we need to develop a method for large scale synthesis of CNTs that ensures consistent shape, size, and function. However, it is evident that our current methods of water purification aren’t enough and motivation is strong to develop this new use for CNTs.6
With increasing resources and technologies, we are closer than ever to a newer, faster, cheaper, and overall better way to provide fresh water on the global scale. Nanotechnology has proven to be a wonderful alternative to previously used methods in energy and medicine, for example, and we may be close to seeing a similar improvement in the case of water purification. As part of the Center for Sustainable Nanotechnology, I want to mention that it will be absolutely vital to produce and dispose of CNTs in a way that doesn’t lead to greater issues in the future (such as their own toxicity to the environment). This is an exciting time in science because we have groundbreaking approaches, but we are also in a day and age where we need to be more thoughtful about how our actions impact our finite supply of water, precious metals, and other vital resources.
EDUCATIONAL RESOURCES
- Teach Engineering: Hands-On Activity: Water Filtration (grades 3-5)
- SafeWaterScience: Treatment of Water Teacher’s Guide (grades 5-8)
- National Nanotechnology Infrastructure Network: The Water Race: Hydrophobic & Hydrophilic Surfaces activity (high school)
REFERENCES (may require subscription for full access)
- The Water Information Program. Water Facts http://www.waterinfo.org/resources/water-facts.
- United Nations Environment Program. Vital Water Graphics, retrieved from http://www.unep.org/dewa/vitalwater/article141.html.
- International Desalination Association. Desalination by the Numbers http://idadesal.org/desalination-101/desalination-by-the-numbers/.
- Texas Water Development Board. Seawater FAQs http://www.twdb.texas.gov/innovativewater/desal/faqseawater.asp.
- Nuclear Energy Institue. Water Desalination
http://www.nei.org/Knowledge-Center/Other-Nuclear-Energy-Applications/Water-Desalination - Das, R.; Ali, M. E.; Hamid, S. B. A.; Ramakrishna, S.; Chowdhury, Z. Z. Carbon nanotube membranes for water purification: a bright future in water desalination. Desalination, 2014, 336, 97-109. doi: 10.1016/j.desal.2013.12.026

[…] bike frame.1 Carbon nanotubes (the same substance from our recent posts about flame retardants and water purification) are a form of hexagonally arranged carbon atoms wrapped into a cylinder. They look a bit like […]