Scientists have known for some time that nanomaterials can stick to cell membranes and, in some cases, damage the membrane in the process. But what exactly do nanomaterials stick to on the cell membrane? A particular type of molecule called lipopolysaccharides (LPS) may provide a key to answering this question.1

Many of us take notice of bacteria only when they make us sick. In reality, bacteria are always working behind the scenes, sometimes making us sick, but more often performing tasks that are beneficial to humans and the broader environment, like helping us to digest our food and supplying nutrients to plants.
As Vivian Feng mentioned in her recent post, there are two ways that bacteria are likely to encounter nanomaterials: when the bacteria are being directly targeted by nanomaterials (as in nanoparticle-based antibiotics), or when they randomly encounter nanomaterials that humans release into the environment.
In either case, it is imperative that we understand how bacteria interact with the nanomaterials that we make: this will help us increase the effectiveness of nanomaterials that are designed to kill bacteria, and decrease the killing-power of nanomaterials that enter the environment as nano-waste, where beneficial bacteria are hard at work. (For more on this, take a look at Part 1 and Part 2 of our series on how nanoparticles get into the environment.)
As I mentioned at the beginning of this post, scientists have known for a while that some nanomaterials can stick to and potentially damage cell membranes.2,3 But knowing that isn’t always enough to give us control over which nanomaterials will be antibiotics and which will be harmless to bacteria in the environment. The cell membrane is not a single uniform entity, but is composed of a sea of molecules including lipids and proteins. We need to identify which of these components is sticky for nanomaterials in order to control how those materials interact with bacteria.
To do this, we (meaning myself and a number of collaborators within the Center for Sustainable Nanotechnology) recently studied how gold nanoparticles interacted with both living bacterial cells (using a bacterium called Shewanella oneidensis) and artificial cell membranes that we made in the lab using the same kinds of molecules found in real cell membranes. Specifically, we varied the amount of one molecule, called lipopolysaccharides (LPS), and observed the impact this had on how sticky the membranes were for the nanoparticles.1
Why did we focus on lipopolysaccharides? This question is best addressed with a picture. As shown below, gram-negative bacteria have two lipid membranes (drawn as sandwiched structures composed of red/pink heads and white/yellow tails). The outer one contains LPS molecules (orange curved lines) that stick out from the cell membrane, kind of like the hooks on Velcro. We hypothesized that this would make the LPS molecules more available than other components of the membrane to stick to things that pass by the cell, like nanoparticles.
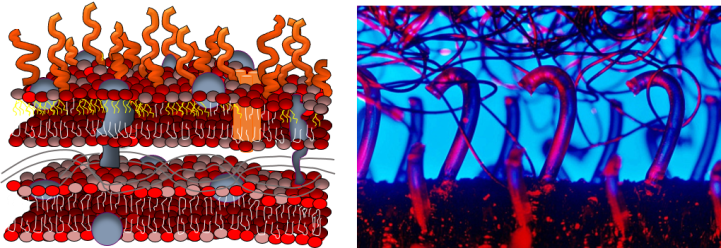
Our results suggest that our hypothesis was correct. When we removed LPS molecules from live bacterial cell membranes, fewer nanoparticles stuck to them. Similarly, when we incorporated fewer LPS molecules into artificial membranes, fewer nanoparticles stuck to them. Bacterial species differ not only in how much LPS is present in their cell membranes, but also in how long those molecules are. We used a type of laser spectroscopy to show that gold nanoparticles that stuck to longer LPS molecules were held farther away from the membrane than nanoparticles stuck to shorter LPS molecules.
So what does all of this mean in the long run? Our work suggests that bacterial membranes richer in LPS molecules may be stickier for some types of nanomaterials, and that those with longer LPS molecules may trap nanomaterials farther away from the membrane, where we think the nanomaterials can do less damage to the cell. We expect that this work will help us (and other scientists) design better nanomaterials, whether for the purpose of killing bacteria or leaving them alone.
This post is part of our ongoing series of public-friendly summaries describing research articles that have been published by members of the Center for Sustainable Nanotechnology. Ian Gunsolus, a doctoral student at the University of Minnesota, was a co-first author on this paper. The article was first published online in July 2015 in Environmental Science & Technology.1
EDUCATIONAL RESOURCES
- ORISE: The Cell Membrane: The Gatekeeper of the Cell lesson plan (grade 7)
REFERENCES (some require subscription for full access)
- Jacobson, K., Gunsolus, I., Kuech, T.; Troiano, J., Melby, E., Lohse, S., Hu, D., Chrisler, W., Murphy, C., Orr, G., Geiger, F., Haynes, C., & Pedersen, J. Lipopolysaccharide density and structure governs the extent and distance of nanoparticle interaction with actual and model bacterial outer membranes. Environmental Science & Technology, in press. doi: 10.1021/acs.est.5b01841
- de Planque, M., Aghdaei, S., Roose, T., and Morgan, H. Electrophysiological Characterization of Membrane Disruption by Nanoparticles. ACS Nano, 2011, 5 (5), 3599–3606. doi: 10.1021/nn103320j
- Liu, S., Wei, L., Hao, L., Fang, N., Chang, M., Xu, R., Yang, Y., & Chen, Y. Sharper and Faster “Nano Darts” Kill More Bacteria: A Study of Antibacterial Activity of Individually Dispersed Pristine Single-Walled Carbon Nanotube. ACS Nano, 2009, 3(12), 3891–3902. doi: 10.1021/nn901252r
