The football community is abuzz about the latest scandal to hit football – deflate-gate! The New England Patriots are accused of intentionally using footballs with pressure lower than that allowed by the NFL in the Championship game last week. Finger-pointers argue that the Patriots intentionally cheated, because a ball with lower pressure is more “squishable” – easier to grab, easier to throw and to catch. The Patriots argue that the balls were filled within regulation pressure before the game, and that the decrease in pressure must have been due to the difference in temperature between when they were filled and when the game took place.
Is this a reasonable explanation? Let’s do the science.

Everybody knows that a gas (and almost everything else) expands when it gets warmer. This expansion happens because the thing we call temperature is a measure of how fast gas molecules are zipping around: higher temperature means more zipping around, and molecules that are moving around faster take up more space. Pressure is the “rebound” when these gas molecules hit an object. At lower temperatures the gas molecules move slower, and so the rebound effect that we call “pressure” decreases too.
The picture below shows what’s happening. Inside the football the gas molecules are moving around, and some hit the interior walls. The little arrows represent the speed and direction of the molecules: a longer arrow means faster movement and a shorter arrow means slower movement. There are also gas molecules hitting the outside surface of the football. Each little rebound generates a little bit of “push” that we call pressure. Because we have pumped extra air into the ball, there are more gas molecules per unit volume on the inside than on the outside, and so there is a pressure difference of ~ 12 psi (pounds per square inch). Now consider what happens when we cool the football. For the moment we’ll assume the material of the football itself doesn’t change, so that its volume stays constant (more on that later).
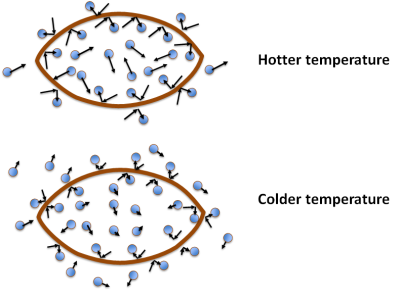
In the lower figure, the gas molecules are moving more slowly – the arrows are shorter – but this has a different effect on the pressure inside of the ball than outside. There are still the same number of gas molecules on the inside as before, which means that when they move more slowly they collide less frequently, and when they do collide with the football the “rebound” pressure effect is weaker. The atmospheric pressure outside of the football, on the other hand, remains constant even though the temperature changes. Each molecule hitting the outside of the ball is moving more slowly too, but at the lower temperature there are more molecules per unit volume on the outside, so the cumulative pressure effect stays the same. (If this wasn’t true, those of us living in winter climates would have the doors and windows of our houses exploding.) So, when we move the football from the locker room to the playing field, the pressure on the outside of the ball remains constant, but the pressure inside the ball changes.
The relationship between temperature and pressure in a closed container is a very simple one and is known to every chemistry student as the “Ideal Gas Law”: PV=nRT, where P is the pressure, V is the volume of the container, T is the temperature, n is the amount of gas in the container (usually measured in units called “moles”, which you may recall from our Mole Day post), and R is a constant known as the universal gas constant. Looks simple, right?
The trick in using the Ideal Gas Law formula is to understand the proper way to represent temperature and pressure. T must be absolute temperature, which is the temperature measured with respect to absolute zero. P must be the absolute pressure, which is the pressure above a perfect vacuum.
The National Football League rules stipulate that the pressure in the ball must be between 12.5 and 13.5 psi higher than the surrounding atmospheric pressure. Atmospheric pressure varies with the weather and other variables, but at the time of the game the nearby Norwood Airport reported the barometric air pressure as 29.63 mm of mercury, which is equal to 14.553 psi, and the temperature at the beginning of the game was reported to be 51oF.
If we assume that the Patriots are telling the truth, we can calculate how the ball pressures would change if they were filled to different pressures and then changed temperatures. Rearranging the Ideal Gas Law, we get P1⁄T1 = P2⁄T2, where P1 and T1 are the pressure and temperature when the ball was filled, and the P2 and T2 are the pressure and temperature when the ball was used on the playing field. Knowing P1, T1, and T2, we can calculate what P2 would be.
Because we need to use absolute pressure and temperature, there are some mathematical conversions that need to be done. We convert temperature from degrees Fahrenheit to the absolute temperature scale (Kelvin), and we convert from “gauge” pressure (pressure measured in excess of normal “atmospheric” pressure) to “absolute” pressure. Instead of showing all of that here, I’ve created an Excel pressure spreadsheet so you can see the conversions and, in fact, you can manipulate the hypothetical ball filling conditions and the playing field temperature yourself.
Using these equations, we predict that if the balls were filled to 12.5 psi at a temperature of 75oF (297.0 Kelvin), and then brought onto the playing field at 51o, the resulting pressure inside the ball would be 11.3 pounds per square inch. Just about 1 psi lower than that minimum allowed by the NFL rules.
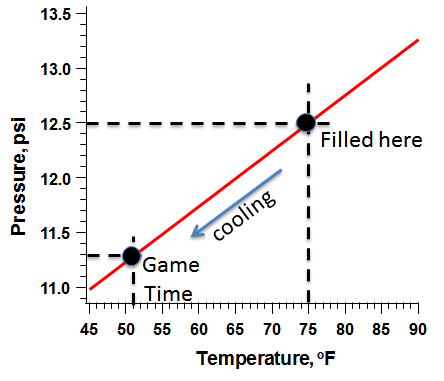
In all of this there are two important assumptions. One is that the football is perfectly rigid and doesn’t shrink when it gets cooler. The other is that the ball doesn’t stretch or relax when the pressure inside changes. In reality, the football itself, like all objects, will shrink a bit as the temperature cools. This has the effect of making the pressure inside higher than it would be otherwise, because the same number of molecules are now confined to a smaller space and so they hit the inside walls more often. In addition, a football has some elasticity — a bit like a balloon, stretching as the pressure inside increases. As you blow more air into a balloon, the pressure of the air inside it goes up a little bit, but not as quickly as it would if you were blowing the same amount of air into something more rigid, like a milk jug. While we don’t know the exact elasticity of a regulation football and we don’t know by how much the football itself expands or contracts with temperature, we do know that both of these effects tend to keep the pressure more constant than it would be otherwise. As the football was brought from the warm locker room onto the cooler playing field, the thermal contraction of the ball and the “balloon effect” would both tend to keep the pressure closer to its original value.
So, did the Patriots cheat? Science says that a pressure as low as 11.3 could be reasonable for a ball that was filled in a modestly warm room at about 75oF and then taken outside into a 51o stadium. It’s also possible the balls were filled at some higher temperature. Could that have been true? If you’re interested, you can download the attached Excel pressure spreadsheet and easily do the calculations yourself. You can put in an assumed temperature and pressure used for filling the balls, and the spreadsheet will show you how the pressure would change at some different temperature.
We’ll probably never know if the Patriots were cheating or not. What we do know is that the Patriots won easily. As a dyed-in-the-wool Green Bay Packers fan my blood runs green and gold and I’d love to be able to accuse the Patriots of cheating. But I’m a scientist, and scientists live by facts. The Ideal Gas Law is a well-established scientific truth, and it tells me that a football filled with air at room temperature could end up below regulation pressure once it got out onto a chilly playing field. However, one would presume that the rulebook refers to the pressure in the ball at the time of play, not hours before under some other set of conditions. Was the Ideal Gas Law an unwilling conspirator in planned deception? Ultimately we’ll never know. What we do know is that, long after the Patriots-Colts game has faded into oblivion, the Ideal Gas Law will still be here, a steadfast truth in the annals of science.

WANT MORE ON THE SCIENCE OF DEFLATE-GATE?
Bill Nye “The Science Guy” on ABC News
The AV Club critiques Neil DeGrasse Tyson’s Tweet
South Florida Sun-Sentinel columnist Dave Hyde does the experiment
UPDATE: MAY 6, 2015
Regardless of the scientific plausibility of the innocent explanation discussed above, the investigative report published today states that, “it is more probable than not that New England Patriots personnel … were involved in a deliberate effort to circumvent the rules.” You can read more at CNN, NPR, or ESPN (among other places).

How about actually doing a real experiment. Inflate a football to 12.5 at room temperature. Put it in a refrigerator for 90 minutes (throw a thermometer in the fridge to get a temperature figure). Then just measure the PSI when you take it out. It will give you a pretty good indication. You might also moisten the ball before you put it in the fridge. The game was played in the rain and leather becomes more elastic when wet. Post the actual results rather than continue speculation. Thanks.
Thanks for the comment – I think you’re right that theorizing, while very informative, can only take us to a certain point. This situation provides a great educational opportunity to explain how the Ideal Gas Law manifests in real life, in an admittedly simplified scenario. Check out the article by Dave Hyde linked above — he did almost the exact refrigerator experiment that you suggest, though without the extra moisture to account for rainy conditions.
Almost everyone comments on the temperature at the start of the game being 50 or 51 degrees. The National Wether Bureau site in nearby Taunton, MA lists the temperature at halftime (which is when the refs checked the pressure) as 44 degrees so from the above graph we would expect 12.5 PSI balls to have fallen to about 11 PSI. You also get some minor contributions from condensation of H2O within the ball at colder temps, and the fact that as the balls became wet in the rain, the leather becomes more elastic (elongation increases). Both of those are probably much smaller effects but would only serve to further drop the PSI. What I have seen is that the theoretical Ideal Gas calculations predict about 1.5 PSI drop and actual testing of footballs is in the 1.5 to 2.0 range.
It will be interesting to see if the NFL admits their mistake or if they will just try to confuse people and hide the scientific proof. We need a Richard Feynman-type moment where he brilliantly performed a simple experiment in front of an audience to show what a temperature drop could do to the Space Shuttle O-ring.
Thanks.
Thanks Bob for the excellent narration. I just prepared a problem for my environmental class based on this analysis.
I also saw this experimental video clip https://www.youtube.com/watch?v=CxsXFX3tDpg
Nice video! And they did soak the footballs a la Wayne’s suggestion above.
Not only is this presently an interesting physical science event, but it will also become a very interesting social science event when the NFL is forced to decide between apologizing to the Patriots or trying to coverup the scientific explanation.