Synthesizing nanoparticles is sometimes a lot like baking cookies. You start with ingredients, mix them together, and put them in the oven. After a few hours, you take them out and admire your hard work. Just like there are lots of different kinds of cookies, there are also many different kinds of nanoparticles. The easiest kind to make is the so-called homogeneous nanoparticle; much like a shortbread cookie, homogeneous nanoparticles are the same all the way through. Like the shortbread cookie, these nanoparticles are very versatile: you can cover them in chocolate (or protein molecules, if we’re talking about a nanoparticle), keep them plain, or use them for any number of other things. They’re not the only kind of cookie, however; my favorite has always been chocolate chip.

There’s another kind of nanoparticle that, like the chocolate chip cookie, is beloved by many because of how different it is than our same-all-the-way-through shortbread cookie example above. These nanoparticles, like chocolate chip cookies, are made up of two parts: 1) the main cookie part, known as the crystal and 2) the chocolate chip part, known as the dopant. The nanoparticle, like the cookie, is mostly crystal, with only a few dopant atoms per particle. If there are too many chocolate chips and not enough cookie to hold the whole thing together, the result is just a mess. But why would we want to add different atoms to our nanoparticle? It’s certainly not for flavor, like the chocolate chips in our cookies are; in this case, it’s so we can “see” the nanoparticles.
Imagine you wake up hungry in the middle of the night, and you remember you made cookies. Of course you’re not going to be able to go back to sleep with cookies on your mind, so you make the trek to the kitchen to get them. Once there, however, you realize the flaw in your plan—it’s so dark in the kitchen that you can’t find the hard-earned treats you’ve been pining for, and you can’t turn on the lights without waking everyone up! If only they made glow-in-the-dark chocolate chips for night-owls like you. Finding and retrieving your dessert would be a cakewalk!

They may not make glow-in-the-dark chocolate chips, but, luckily for us, they do make glow-in-the-dark atoms. Some atoms, when they absorb light of a certain energy, re-emit light of a different energy. This is how TV’s, fluorescent lightbulbs, and various other kinds of modern electronics work. And as anyone who’s ever seen the stars in the sky (or a picture of the Earth at night) knows, things that light up, even from great distances, are easier to see than things that don’t.
The same principle applies to nanoparticles: they are, of course, very small and hard to see, except under very controlled conditions and by very sensitive instruments, like Scanning Electron Microscopes. If you can make the nanoparticles glow, or fluoresce, however, they become much easier to see and detect, even in much less well-controlled environments. If we can make our particles fluoresce when we shine light on them, we can detect them much more easily. Fluorescent nanoparticles are very powerful, as you can put them into things such as cells and organisms and track their movement.
So, one way to make fluorescent nanoparticles is to insert fluorescent atoms into the nanoparticle structure. There are two main ways to do this. I’m trying both of these in the lab right now, and I’ll describe them both below.
Method 1: Ion Incorporation
Doping a crystal with a fluorescent atom is sometimes done in the same way that cookies are “doped” with chocolate chips; the dopant ion can sometimes be put into the crystal “dough” and, as the crystal grows, the dopant is incorporated into the crystal. This method is fantastic if it works, but unfortunately it doesn’t always work.
Growing a crystal is a lot like stacking a lot of tennis balls into a tidy shape, and doping a crystal is like trying to put a different ball into your tidy shape. If your “dopant” ball is a baseball, you probably won’t have too much trouble finding a place in your pile for it, as it is nearly the same size as the tennis balls. If your other ball is a basketball, though, or a ping-pong ball, it will be almost impossible to incorporate it into your pile without the pile becoming unstable and toppling.
Method 2: Ion Implantation
If the atom you’re trying to fit into your crystal is too big or small to “fit” into the crystal, it won’t be incorporated, and your chocolate chip cookie recipe will instead end up making just plain, chocolate-less cookies and a pile of chocolate chips that’s off to the side. In these cases, we need a more direct, forceful approach to doping our crystals.
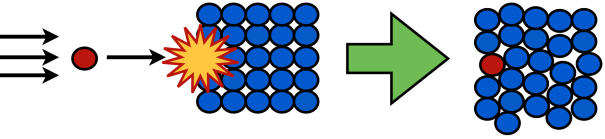
If our dopant ion won’t go into the crystal on its own, we can force it in, a process known as ion implantation. In this method, a homogeneous crystal is formed first. Then, in the implantation step, an ion is accelerated and shot into the crystal, impaling the crystal’s surface and forcing in the dopant atom. The force of the impact can “break” the crystal a little bit, but if there’s no other way to get the ion in, the process can be worth it.
In my research this summer, I’m trying to use both of these techniques to make titanium dioxide, zirconium dioxide, and cerium dioxide nanoparticles doped with europium (one of the most fluorescent) atoms. None of these nanoparticles are easy to track in biological or environmental systems, but if I can make them fluorescent, they will be relatively easy to see using common laboratory techniques and equipment like the fluorescence microscope. Since I’m not sure which of these methods is best, I’m working on both. Hopefully one (or both!) of these is successful, and my research makes it easier to track and study nanoparticles in the environment.

