Half a century ago, a Soviet scientist was tucked away in a top-secret facility. The results of his experiments are critical to our work here the Center for Sustainable Nanotechnology. Find it hard to believe? Read on!
Why We Use Nanodiamonds
One of the materials that we are using in our research is nano-sized diamond. We also call them diamond nanoparticles, or nanodiamond. One of the unique properties of diamond is that it is extremely stable, which allows us to study the behavior of diamond nanoparticles without having to worry about them decomposing.
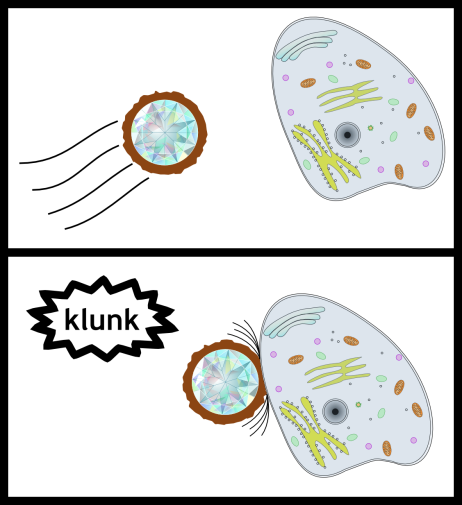
We can also intentionally modify the surfaces of diamond nanoparticles and investigate how they interact with different types of cells and aquatic life. Do the nanoparticles stick to the cells? Do they pass through the cells?
A collection of pure diamond nanoparticles is a grayish looking powder – nothing like the gleaming diamond you might have imagined.

In water the nanoparticles are clear, but we can intentionally make them fluorescent. That way when we shine light of one color on the diamond nanoparticles they will give off light of a different color. Even if the nanoparticles are too small to see by eye, we can still track them using the light they emit.
So we’re using these nanodiamonds to learn to predict how nanoparticles interact with cells and organisms – all this to help create a more sustainable world by taking advantage of the many unique properties of nanoparticles. This is all wonderful, right?
Nanodiamond Formation
Well, in reality it’s more complicated. In fact, when you look deeper, the story of nano-diamond is a complicated one that starts back to the 1950’s and continues into the present day with the threat of nuclear weapons in Iran….. Sound intriguing? Then read on …
Diamonds are pure chunks of carbon atoms, all arranged in a specific way. The challenge of making diamond at room temperature and pressure is that the carbon atoms would rather be in a different arrangement—that of common graphite, the stuff in your pencil. However, if you squeeze and heat carbon, then the atoms can prefer the arrangement of diamond. This is described in something called a phase diagram. Here’s the phase diagram for carbon.
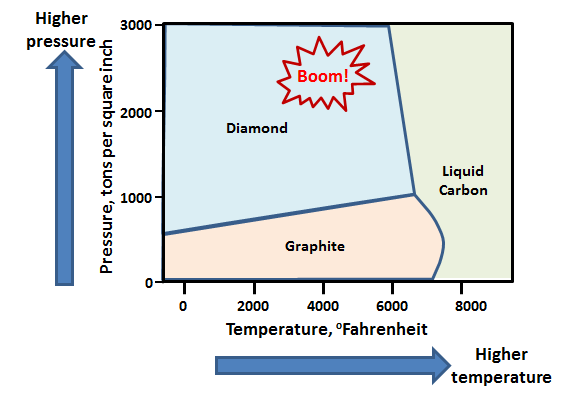
So, one way to make diamond is to heat carbon up to tremendously high temperatures and pressures. How do you create these conditions? There are several ways. In the bowels of the earth the temperatures and pressures are high enough for nature to make diamonds that are then ejected from volcanos in a mineral called Kimberlite. If you’re on the surface of the earth though, there’s a different way…. Bombs.
Detonation Nanodiamonds
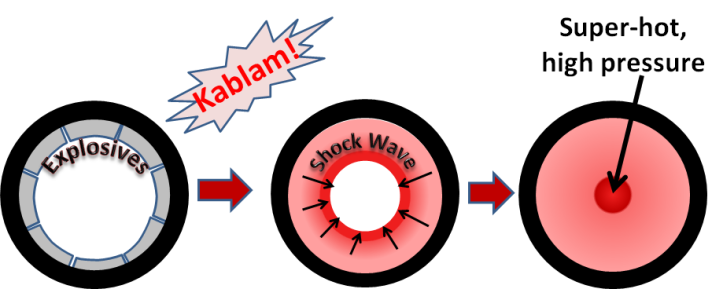
Detonation nanodiamonds were first made in 1963 by three scientists: K.V. Volkov, Vyacheslav Danilenko, and V.I. Elins. They worked at the All-Union Research Institute of Technical Physics in the Soviet Union. Located in the “secret” city of Chelyabinsk-70 , this facility was for doing research on nuclear bombs. In order to make a nuclear bomb it is necessary to very rapidly squeeze the “core” of the bomb. The easiest way of doing this is to surround the core with explosives and blow them up.
The Soviet scientists were using carbon-containing explosives like the one below.
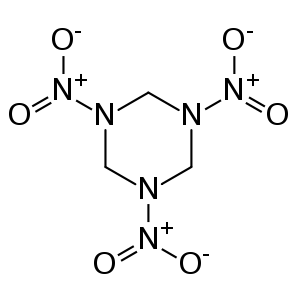
Around 1962, they discovered that the temperatures and pressures in the shock waves of these explosions were high enough to squeeze the carbon atoms from explosives residue into diamond nanoparticles. For many years the Russian scientists conducted studies of nanodiamond synthesis, but most of this work was kept secret because the same methods used to make nanodiamonds would also be directly related to their nuclear bomb technology.
The Secrets are Released
When the Soviet Union dissolved in 1991, their huge bomb development efforts stopped and the information about how to make nanodiamond became public knowledge. Nanodiamond is now used by researchers around the world. Instead of being made at secret military sites, it’s made in commercial high-pressure reactors and can be bought on-line with a credit card.
Unfortunately, the “Swords into Plowshares” story doesn’t end there. With the end of the Cold War in 1991, many of the Russian scientists who had worked at Chelyabinsk-70 and other Soviet military research facilities found themselves out of work. Vyacheslav Danilenko, one of the three scientists involved with the initial nanodiamond work, attempted to commercialize nanodiamond in Europe and the US, but he was not successful. Around 1996, Danilenko was hired by Iran as a consultant. What happened next is murky, but is outlined in an article in the Washington Post and a 2011 report from the International Atomic Energy Agency (IAEA).
The IAEA report says that a “foreign expert” (presumably Denilenko) went to Iran in 1996, and that this expert claimed he went there to help establish a facility for making detonation nanodiamond. While there he also gave lectures on explosion physics and its applications. These lectures would be of particular interest to anyone trying to make a nuclear bomb. A few years later, satellite images show the construction of a large explosion chamber – like that used to make nanodiamond, but also perhaps capable of testing detonators for nuclear weapons.
Did Danilenko, one of the inventors of nanodiamond, use his knowledge of controlled explosions to help Iran develop its nuclear bomb capability? The story is unclear. Danilenko may well have had entirely peaceful intentions. But did his expertise help others accelerate Iran’s nuclear abilities? It’s likely that we will never know. Danilenko left Iran in 2002, and according to the Washington Post he now works in the Czech Republic for a company called Nanogroup that is, once again, attempting to commercialize the synthesis and application of nanodiamond.
Let’s hope that this effort is successful and that we can turn swords into plowshares, instead of turning plowshares into swords. We’re certainly trying to do our part to use nanodiamond for peaceful purposes—to further the sustainability of our global community.
Further reading and references:
1. V.V. Danilenko, “On the History of the Discovery of Nanodiamond Synthesis”, Physics of the Solid State, 2004, vol 46, pp. 581-584.
2. Russian scientist Vyacheslav Danilenko’s aid to Iran offers peek at nuclear program
3. Iran nuclear report: IAEA claims Tehran working on advanced warhead
4. ISIS Reports: Vyacheslav Danilenko – Background, Research, and Proliferation Concerns


[…] Na verdade, uma pilha de nanopartículas de diamante puras parece ser só um monte de pó meio marrom, meio cinza. Imagem via Sustainable Nano […]
[…] Na verdade, uma pilha de nanopartículas de diamante puras parece ser só um monte de pó meio marrom, meio cinza. Imagem via Sustainable Nano […]
[…] Na verdade, uma pilha de nanopartículas de diamante puras parece ser só um monte de pó meio marrom, meio cinza. Imagem via Sustainable Nano […]
[…] Na verdade, uma pilha de nanopartículas de diamante puras parece ser só um monte de pó meio marrom, meio cinza. Imagem via Sustainable Nano […]
[…] our Center is made via the top-down approach. Nanodiamond is nearly pure carbon. Nanodiamond is made by detonation (explosion) or ultrasonication (using sound waves to break up materials) of bulk graphite […]
[…] A pile of pure diamond nanoparticles actually just looks like agrayish-brown mound of dirt. Via […]