During a recent visit to Home Depot I came face to face with a lithium-ion battery, nanotechnology, and my interest in making a wooden-framed mirror.

I had my eyes on some shiny, new Dewalt power tools. I decided that it would be much easier to build a wooden frame with a high quality drill and router. And besides, a wooden-framed mirror is more expensive than both tools combined.
I may have left the store empty handed, but I continued to do some research online. I found out that the drill I so dearly needed actually contains nanomaterials in its lithium-ion battery! As a chemist interested in nanotechnology, I was intrigued—I couldn’t believe that I was unaware of nanotechnology being used to power the drill I was just looking to buy!
I decided to search around a bit more to see how and why nanotechnology is being used to improve lithium-ion batteries.
If you are reading this blog by means of an electronic device, such as a laptop or cell phone, it is highly likely that the device you are using is also powered by a lithium-ion battery. These rechargeable batteries are extremely popular and versatile and can be found in many different types of electronic devices from computers to cars and of course, power tools. Even NASA makes use of the lithium ion battery in space!
But why are these lithium-ion batteries so widely used in electronic devices and how is nanotechnology being used to improve this technology?
Let’s start off with how the lithium-ion battery works. Batteries store and releases energy by moving electrons from one “end” of the battery to the other. Then we can use the energy from those moving electrons to do work for us, like power a drill. These two battery “ends” are known as electrodes. One is called the anode and the other is called the cathode. Generally, the anode is made from carbon and the cathode from a chemical compound known as a metal oxide (cobalt oxide, for example). The final battery ingredient is known as the electrolyte, and it sits in between the two electrodes. In the case of lithium-ion batteries, the electrolyte is a salt solution that contains lithium ions—hence the name.
When you place the battery in a device, the positively charged lithium ions are attracted to and move towards the cathode. Once it is bombarded with these ions, the cathode becomes more positively charged than the anode, and this attracts negatively charged electrons.
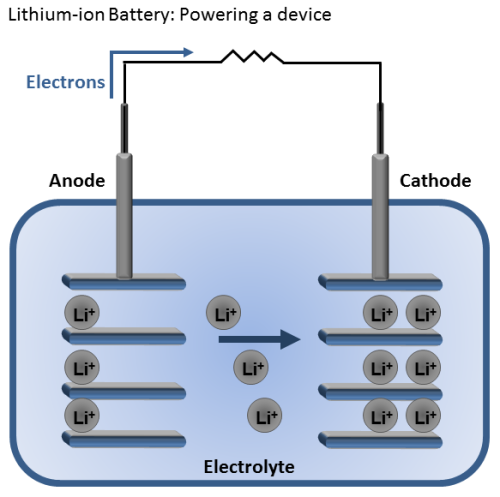
As the electrons start moving toward the cathode, we force them to go through our device and use the energy of the electrons “flowing” toward the cathode to generate power. You can think of this kind of like a water wheel, except instead of water flowing, electrons are flowing.

Lithium-ion batteries are great because they are rechargeable. When the battery is connected to a charger, the lithium ions move in the opposite direction as before. As they move from the cathode to the anode, the battery is restored for another use.
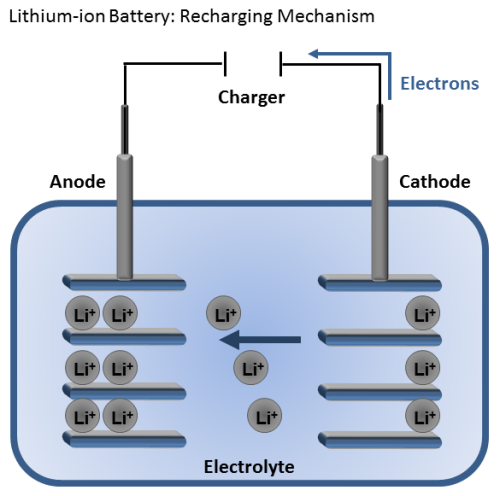
Of course the fact that the lithium ion battery is rechargeable makes it more desirable and sustainable, but why else are these batteries so widely used?
One reason is that lithium ion batteries can produce a lot more electrical power per unit of weight than other batteries. This means that lithium-ion batteries can store the same amount of power as other batteries, but accomplish this in a lighter and smaller package.
However, one downside to lithium-ion batteries is that they take much longer to charge than other batteries. And of course, there is always room for improvement in efficiency. This is where nanotechnology comes in. In order to improve the efficiency and decrease the charge time of lithium-ion batteries, many companies and researchers are using nanotechnology to make better battery materials.
A lot of research is focused on using nanotechnology to make better electrodes. Using nanomaterials in the electrodes increases their surface area, which provides more places for the lithium ions to make contact. This makes the battery more efficient and also makes it recharge faster. These changes should make electronic devices that use lithium-ion batteries (e.g. laptops), lighter, and also allow them to go a longer time before recharging.

Check out the lithium-ion batteries used in your electronic devices—has nanotechnology been implemented into those devices yet? Click here for a cool search tool from the Project on Emerging Nanotechnologies. Please note that, as Christy pointed out in her post, companies are not required to label their products as being nano-enabled, so that list is definitely incomplete.
References & Further Reading:
Reliability ‘key’ to space-qualified li-ion batteries
Nanophosphate lithium ion battery technology – white paper request form
Nanophosphate lithium iron phosphate battery technology
Electronically conductive phospho-olivines as lithium storage electrodes. Nat. Mater. 2002, 1, 123-128
Post edited Sept 19, 2023 to correct typo in the last image.

Wow, wonderful blog structure! How long have you ever been running a
blog for? you made running a blog glance easy. The whole glance of your site is magnificent, as smartly as the content material!
kids doing this project at school fair. See more: http://mocomi.com/how-do-batteries-work/
Rohit
Cheers for the post, Julianne. While we’re on the subject of lithium-ion technology, I came across an interesting article detailing the research work by a group of Korean scientists who have developed a possible use of Rice (yes, Rice!) in li-on batteries. Read more about it here:
http://firstlook.pnas.org/battery-materials/