You’re looking at an electron micrograph of gold nanoparticles; a snap shot of tiny gold crystals that are 1/10,000th the diameter of a human hair. Nanoparticles just these like may soon transform every aspect of our day to day lives, from the size of our personal electronic devices to the way diagnose and treat cancer; all part of the promised nanotechnology revolution. The very word “nanotechnology” seems to suggest something alien; something that belongs far in the future or in the realm of our favorite sci-fi movies. But nanoparticles, one of the “building blocks” of nanotechnology are all around us right now, and have been all around us throughout human history. They were with us when human beings began making their first tools, and they are present in products we buy at the grocery store every day. They largely flew under the radar until electron microscopes become commonplace several decades ago, but now, the more we turn our microscopes on everyday objects, the more nanoparticles we seem to find.
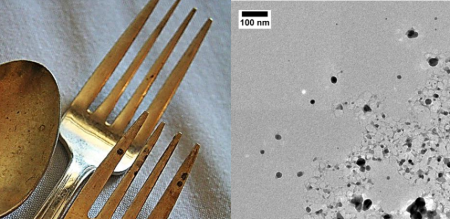
Even the most seemingly mundane objects can give rise to nanoparticles; detecting them is simply a matter of being able to look closely enough to see them (no simple matter for such small materials). You could find nanoparticles in your jewelry box or the drawer with your family’s fanciest silverware. I got to see this first hand while I was working in the Hutchison lab at the University of Oregon several years ago.1 Some of my colleagues were trying to understand why silver nanoparticles change size and shape so rapidly, even when they are just left in storage on the shelf. Because they saw such rapid changes in the size and shape of silver nanoparticles, they thought to look and see if large every day pieces of silver and copper (Sterling silver forks, earrings, and wires) might give off nanoparticles.2 To test this, they simply left the fork (or any of the other items) on an electron microscopy grid for several hours, then took the fork away, and had a peek at what it had left behind. Surprisingly, they found that the silver and copper items had left silver and copper nanoparticles behind all over the grid; a most elegant demonstration that human beings can come into contact with a variety of nanoparticles, even in our own homes. Forks and earrings are merely the tip of the iceberg, though. Wherever we go during our day-to-day routine we can encounter nanoparticles (both synthetic and natural).
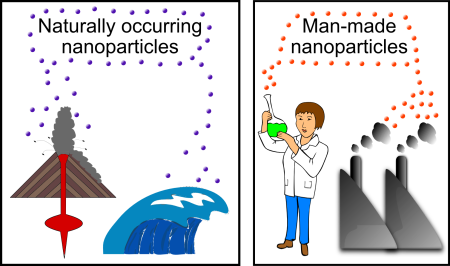
It seems weird to say, but (as the fork study shows) nanoparticles are actually a fairly common type of material in many different environments, and they can pass by almost undetected unless you are looking for them. In fact, many kinds of physical and chemical processes (both human activities and natural processes) produce nanoparticles. Naturally occurring nanoparticles can be found in volcanic ash, ocean spray, fine sand and dust, and even biological matter (e.g. viruses). Synthetic nanoparticles are equally, if not more diverse than their naturally occurring counterparts.
Synthetic nanoparticles (sometimes called anthropogenic nanoparticles) fall into two general categories: “incidental” and “engineered” nanoparticles. Incidental nanoparticles are the byproducts of human activities, generally have poorly controlled sizes and shapes, and may be made of a hodge-podge of different elements. Many of the processes that generate incidental nanoparticles are common every day activities: running diesel engines, large-scale mining, and even starting a fire.

Engineered nanoparticles on the other hand, have been specifically designed and deliberately synthesized by human beings. Not surprisingly, they have very precisely controlled sizes, shapes, and compositions. They may even contain “layers” with different chemical compositions(e.g. a core made out of gold, covered in a shell of silica, and coated with specifically chosen antibodies). Although engineered nanoparticles get more sophisticated with each passing year, simple engineered nanoparticles can be created by relatively simple chemical reactions that have been within the scope of chemists and alchemists for many centuries. This means that long before people could “see” a nanoparticle through an electron microscope, human beings were both deliberately and accidentally generating a wide variety of these materials.

It’s hard to say when human beings started making incidental nanoparticles, but probably as soon as people started taming fire—you can find small nano-scale particles of soot in the embers and smoke. Certainly, by the Bronze Age, incidental copper nanoparticles would have been prevalent in human civilizations. The earliest engineered nanoparticles are often attributed to the ancient Romans, Egyptians, and Chinese. Despite not having any idea about the scientific implications of what they were making, they were successfully able to prepare nanoparticle solutions of gold and other precious metals with reasonably precise control over particle size and composition. In fact, some of the methods used in these ancient cultures to make nanoparticles were very similar to the way many metal nanoparticles are still synthesized in laboratories today. These antiquarian gold nanoparticle solutions were vibrantly colored (generally reds and purples) and could be impregnated into glass to make stained glass and jewelry. In addition, many people ingested these colloidal gold and silver solutions as health tonics; specifically to treat high fevers and syphilis. Though it’s unlikely that drinking metal nanoparticles has any truly positive aspects for your health, some people still swear by this folk remedy, with often unappetizing, though not necessarily lethal, results.

As human society has advanced technologically, it is likely that exposure to all kinds of synthetic nanoparticles has dramatically increased and will continue to increase. The advent of the industrial revolution provided an opportunity for human beings to be exposed to the byproducts of mining and fossil fuel burning on a scale never before seen. Indeed, the prevalence of black lung in miners for the past several hundred years can be linked to the respiration of ultrafine (aka nano) particles, and even today, people living near high-traffic areas/large-scale manufacturing operations often experience a much higher incidence of chronic respiratory problems that are beginning to be linked with nanoparticles found in diesel exhaust. Ever since researchers began to consider nanotechnology to be a unique research field, however, we have also seen a sharp rise in consumer products that deliberately include synthetic nanoparticles. These nano-enabled products range from cosmetics to sporting goods, and the nanoparticless they contain serve a variety of functions: everything from acting as UV-blocking agents to making plastics that are extremely light, but stronger than steel. Some of these nanoparticle-enabled products actually made it onto the market before people recognized that their benefits were specifically related to the nanoparticles that they contained (e.g. silver impregnated wound dressings). The Woodrow Wilson Institute supports a database of ordinary consumer products that contain nanoparticles, and they now list more than 800 registered nanoparticle-enabled products available to consumers.
Perhaps the easiest place to find nanoparticles in your very own home is in health care products and cosmetics. A number of companies have, for at least the past decade, sold sun block that contains combinations of of zinc oxide (ZnO) and titanium dioxide (TiO2) nanoparticles because these materials absorb UV-light very strongly, preventing your skin from feeling the unwanted effects of ultra-violet exposure. The smaller these particles are, the easier it is to keep them evenly mixed in the liquid portion of the sunblock. This means you are less likely to notice the chalky bits of semiconductor on your skin and (most importantly, of course!) the less likely you are to look like you are covered in chalk. Therefore, using TiO2 and ZnO nanoparticles is an excellent way to ensure comfortable and uniform sun protection.
Clearly, it’s not that hard to find nanoparticles anywhere we go in our everyday lives. We inhale them in tiny amounts as we go about our day-to-day activities, we buy products that use nanoparticles to protect our health, and even objects as mundane as a fork leave behind nanoparticles as they sit unused in drawers. Until recently, we haven’t really noticed how often we are exposed to nanoparticles, because they can be extremely difficult to detect (unless you are specifically looking for them with the right equipment). One question this raises (and I wish we had time to explore it in detail today) is: “Is this ever-increasing level of nanoparticle exposure good for us?” Unfortunately, as Stephanie previously pointed out, we have no easy answer to that. As it happens, some of the nanoparticles that you are exposed to everyday that can be harmful to you (particularly the nano-sized particles that can be found in certain types of pollution) but others have already become our go-to weapons to prevent disease (whether that disease is skin cancer or bacterial infection). So maybe the amount of nanoparticle exposure is not the most important question to ask. Maybe it would be more useful, instead, to ask: “Can we come up with some guidelines that allow us to ‘spot’ which nanoparticles benefit human health and which nanoparticles will potentially do the most harm? And can we tailor our exposure to both accordingly?” We’re working on answers, so stay tuned!
Footnotes
1 I’ve over-simplified some aspects of this study for narrative convenience. This study definitely required some really painstaking experiments. If you follow the link, you will see some fantastic videos of silver nanoparticles changing shape. Special credit must be given to a really good scientist (Rick Glover) who, to make these videos and do the stats for this study, had to take hundreds of electron microscopy pictures of exactly the same particles day after day after day. A full account of the study is here (requires ACS Journal access).
2 Chemists, physicists, and material scientists usually call big chunks of metal “bulk metal.” “Bulk” means that the materials act like we expect ordinary, everyday materials to behave; in general, materials with “nano” dimensions behave very differently from bulk materials. That’s why nanomaterials are so cool.
Note: This post was updated on Sept 10, 2015, to change the term “man-made” to “synthetic.”